1. Introduction
Nematodes are a diverse clade of organisms that infect many species, including vertebrates and invertebrates. Entomopathogenic nematodes (EPNs) are a natural threat to insect larvae and are of interest in the agricultural industry regarding pest control. EPNs parasitize Lepidopteran species as well as
Drosophila larvae, which in turn serve as a model for understanding the host immune response against nematode infections in general, like in the case of Elephantiasis or Onchocerciasis. They enter the host through either the mouth or anus or use their hook-like tooth to burrow into the cuticle, past the epithelial layer to reach the hemocoel. Here, they are able to complete their life cycle, reproduce, and cause septicemia in the host through regurgitation of their symbiotic bacteria [
1]. There are two genera of EPNs that are frequently used in larval infection assays,
Steinernema and
Heterorhabditis. Different species of
Heterorhabditis and
Steinernema are known to either ambush their hosts in order to gain access to the hemocoel, or actively seek their host, or “cruise” when the host is more likely to be sedentary [
2,
3]. In addition,
Steinernema have been reported to be more pathogenic to the
Drosophila host [
4]. Perhaps one reason these EPNs have different infection strategies and pathogenicity is that they are not closely related and underwent divergent evolution, including their parasitization strategy [
5,
6].
EPNs form a complex necessary for proliferation after infection, which entails a symbiotic relationship with gram-negative proteo-gamma bacteria, such as
Photorhabdus spp. and
Xenorhabdus spp. in
Heterorhabditis spp. and
Steinernema spp. nematodes, respectively [
7]. After entry, the worms regurgitate their bacteria into the host. Once bacteria proliferate, this signals to the dormant infective juvenile to differentiate into the sexual-stage adult. The bacteria release toxic secretions, which aid in the EPN complex invading the host, suppressing the immune function, and breaking down the internal tissues [
8]. While bacteria provide nourishment for the nematode, axenic nematodes can successfully infect and kill host larvae, though with lower mortality rates [
1]. It has also been documented in
Galleria that the hemocytes do not attack invading EPNs and that PPOs are suppressed by the EPN complex, which can be seen in the decreased levels of melanization [
9,
10].
In the presence of EPNs,
Drosophila larvae change their behavior, indicating their ability to detect the presence of external threats and engage in potentially protective behavior [
11,
12]. Nevertheless, EPNs can infect
Drosophila either through cuticle or gut penetration, although it is unknown if there is a mode of entry that is more accessible or preferred for some EPNs, such as
Heterorhabditis. However, when infected larvae are entered by an EPN via the cuticle, several pathways that are developmentally and immune associated are differentially regulated as compared to a control larva, such as the Hedgehog, Wnt, and JAK/STAT pathways. Several proteins of interest in immune function and wound healing are also observed to be specific to the anti-nematode response, which includes the involvement of a complement-like protein, TEP3, a basement membrane component, glutactin, and a recognition protein, GNBP-like 3 [
13,
14]. Nematode infection has also been determined to be inhibited by several proteins that are clot-associated, such as transglutaminase, IDGF2 and 3, and fondue (see FlyBase.org for more gene details [
15,
16,
17]).
While details of Heterorhabditis and Steinernema infection in Drosophila melanogaster have been explored in several studies, including important humoral and cellular components of host immunity, the exact mechanism of entry, subsequent proliferation in early stages of infection, as well as the determination of an advanced point of infection have not yet been fully elucidated. In this study, we used high-resolution microscopy to gain further knowledge on the mode of entry and proliferative events of Heterorhabditis bacteriophora and Photorhabdus luminescens. We also distinguished early infection from advanced infection at a time point at which bacterial sepsis overcomes the host and eventually leads to host mortality. We found that EPNs enter through the cuticle of the host with a twisting action, resembling the mode of entry of other parasites, as well as determined the timing of septicemia, loss of tissue integrity, and specific nematode titers, which will lead to septic events. With these data, we provide increased temporal and spatial infection kinetics, which can further increase our knowledge regarding a threshold for overcoming the host immune system, which has implications for both the agricultural industry and human health.
3. Results
3.1. Mode of Entry of Heterorhabditis into the Drosophila Cuticle
Entomopathogenic nematodes (EPNs) are known to enter the host either through the cuticle or through open orifices, with certain species of EPNs preferring one mode or both modes of entry. In the case of
Heterorhabditis bacteriophora, it has been documented to enter through the mouth and anal cavity as well as by penetrating the cuticle [
14]. However, the exact mechanism of entry through the cuticle and exsheathment has not yet been observed for
Heterorhabditis bacteriophora. In order to follow the EPN
H. bacteriophora in its effort to gain entry into the hemocoel of
Drosophila larvae, we used a combination of
H. bacteriophora and its symbiotic counterpart,
Photorhabdus luminescens, which contained a GFP-expressing plasmid [
1]. We exposed w
1118 larvae to high titers of EPNs, 500 IJs/10 μL/larva, for 30 to 45 min until larvae were swarmed with many EPNs; EPNs preferred some individual larvae compared to other nearby larvae. A larva that had not yet been infected but was swarmed, as determined by an absence of a GFP signal within the cavity of the larva, was selected, unwashed, and carefully placed onto a 13-mm cover glass with superglue. Time-lapse microscopy was subsequently used to observe EPN behavior upon penetrating the host.
In
Figure 1, we saw several EPNs adhered themselves to the cuticular surface of the larva; however, only one would go on to enter the larva (frame 1, nematode of interest outlined in red). The nematode of interest engaged in a twisting motion in order to position itself for entry (frame 2–6), and after about 45 min, it finally disengaged its inner from its outer cavity (frame 9–10), leaving behind an empty sheath that stayed appended to the outside of the host cuticle. The IJ then entered the host and started to regurgitate bacteria from the point of origin (frame 10–12;
Figure 1A time series, outlined larva; see also
Movie S1, a time-lapse and 2, a 3D reconstruction of the entry moment with a focus on the nematode of interest). Although this larva had around 16 EPNs attached to the cuticle, only one successfully entered the cuticle while another EPN, pictured in frame 8 (red filled arrow), maneuvered itself violently enough until it is seen detaching in frame 9 (see arrows). Another EPN (the empty red arrow) is seen throughout the time-lapse partially inserted into the host cuticle while never successfully gaining entry.
Of note in Frame 12 in
Figure 1A is the bacterial dissemination from close to the point of entry towards the anterior end of the larva—rather than being taken up into the larva’s open circulatory system, the bacteria appear to attach to the walls of the organ and spread towards the anterior end of the larva. These data support previous evidence for a functional compartmentalization of the hemolymph upon injury [
24]. A schematic of the point of entry shows the nematode at first attached to the host’s cuticle; then it dislodged its inner cavity from its outer sheath, which allowed it to finally enter the host with its symbiotic GFP-expressing bacteria (
Figure 1B). Using the image processing software, IMARIS, the nematode of interest was tracked according to its GFP pixel intensity and spatial relation (z plane coordinates) in order to determine if the EPN was either above or below the cuticular barrier of the host (red line,
Figure 1C; based on
Movie S2). The GFP signal concentrated from the middle of the EPN to the tip of the barrier between the EPN and the larva (shown with the red line on the graph); the signal descended through z positioning over time as the inner cavity of the EPN entered the inner side of the host.
The drill-like motion for entry into the host may demonstrate convergent evolution with other parasite species, such as
Toxoplasma gondii [
25]. The speed of entry of the outlined EPN and the low success of entry of other EPNs in these frames could be the result of the larva being immobilized to the cover glass, rendering the infection scenario not ideal for the nematodes; thus, a certain threshold of entry was needed in order to finish the entry process. Once exsheathment occurs, which exists to protect the worm from harsh environments, the infection process can be carried out [
26].
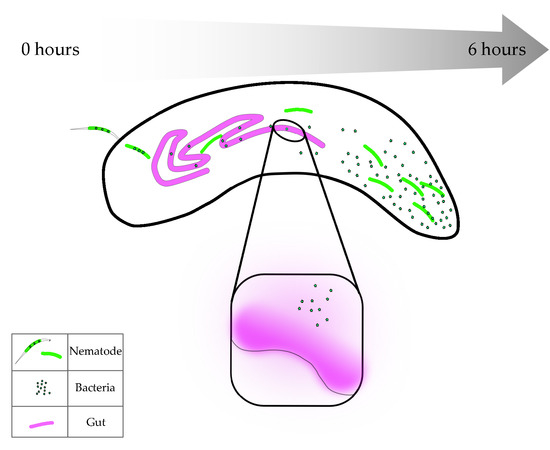
3.2. Tracking EPN Trajectory within the Host
Some parasites require specific tissues or environments within the host in order to differentiate into different stages of their life cycle, as is the case with
Plasmodium falciparum travelling to hepatocytes to go from a sporozoite to a merozoite [
27]. As EPNs will differentiate into the sexual stage after about 24 h, we wondered if there was a region where EPNs would be most likely to be found within the host cavity once an infection occurred. In order to determine if there was a predictable behavior of nematodes within the host cavity, we tracked
Heterorhabditis via their GFP-expressing symbionts and created heat maps of time lapses that were taken from 1 to 6 h after infection. We found that EPNs seemed to spend a majority of time in the anterior end of the larvae (
Figure 2A–C). In a low infection scenario, 1 to 4 EPNs, the pixel intensity was demonstrated to be the highest in the anterior end of the animal (
Figure 2A). In a medium infection scenario, with 5 to 10 EPNs, a more distinct track was found to be travelling along the dorsal side of the animal from posterior to anterior and similarly showed a higher pixel intensity of GFP at the anterior end (
Figure 2B). Finally, in a high infection scenario, with greater than 10 nematodes, a very distinct track is found along the dorsal end towards the anterior side (
Figure 2C).
Thus, even across different infection intensities, nematodes behaved similarly across different infection loads and migrated towards the anterior region primarily. In several high infection scenarios (
Figure 2C and data not shown), nematodes were seen to move from within the body out through the oral cavity and occasionally transgressed through the oral cavity in both directions. Therefore, the movement of nematodes within the cavity did not appear to be random. Further exploration and understanding of the infection biology are necessary to determine if this behavior has a role in the EPN’s ability to overcome the host’s immunity and subsequently differentiate into the sexual stage.
3.3. Rate of Bacteria Proliferation after EPN Infection
After entry into the host hemocoel, EPNs regurgitate their bacteria, which will subsequently proliferate in the host cavity. However, it is unknown how rapidly the nematodes regurgitate their gut bacteria,
Photorhabdus luminescens, and how long subsequent bacterial proliferation takes to cover the host cavity and cause sepsis. We also wondered how different titers of nematodes could affect the rate of bacterial proliferation. We observed in
Movie S1 that after entry into the host, bacteria were released close to the point of entry and progressed throughout the larval cavity from the posterior to anterior end in about 6 h. Thus, we decided to compare the proliferative events of the bacteria during the first 6 h of infection. We infected larvae and selected for either “light,” infection loads of 1 to 4 EPNS or “medium” infection loads of 5 to 10 EPNs. After larvae were infected with the EPN(s) for 1 h, time-lapses commenced. Bacterial GFP pixel intensities were compared at “t0”, or 1 h after infection, until “t5”, or 6 h after infection. Across light and medium infection, the GFP intensity, or mean grey value, increased over time (
Figure 3A). We found that bacterial proliferation was three times higher in the six-nematodes infection scenario than the one-nematode infection scenario after 5 h of time-lapse imaging (
Figure 3B).
The number of EPNs inoculating the larva with bacteria sped up the infection process by 3 times. Furthermore, the immune responses of the larvae may vary depending on the inoculation load of the larva. With this data, we determined a window for the infection period in which the immune response likely gets mounted and subsequently overcome. These are important data to consider when comparing individual larval immune responses to one another in genetic studies; different rates of bacterial proliferation will likely lead to varying rates of sepsis as well as immune induction responses.
3.4. Sepsis and Multiplicity of Infection
As we observed that bacteria were able to cover the host cavity within 6 h in
Figure 1 and
Figure 2, we hypothesized that a loss of tissue integrity occurred in this time, which would lead to sepsis within the host. To determine the loss of gut integrity, we fed larvae for 2 h on 4 different pH dyes. The pH dyes covered ranges across the whole pH scale to determine if virulence factors secreted from bacteria used changing ion concentrations to create a more favorable environment for replication and eventually host death. We used 4 pH indicators as well as a regular cell stain, Crystal Violet Solution, to visualize the dye better and to control against indicators causing the tissue lysis (
Figures S1 and S2). When color was observed in the open circulatory system of the larva, the larva was dubbed a Smurf, which in our scenario represented septicemia [
18,
20]. We found that EPNs did not affect gut pH when releasing virulence factors (data not shown) and that gut integrity, as detected with bromothymol blue, remained intact after 2 h, started to deteriorate by 4 h, and had largely deteriorated by 6 and 8 h (
Figure 4A,B). In
Figure 4A, at the 2-h time point, an EPN was seen inside the larva that had not yet released its symbiotic bacteria (arrow) while at the 6-h time point, sepsis was observed after the bromothymol blue turned from blue to yellow (hemolymph has a pH of 7, see
Figure S1 for pH details).
Since
Drosophila larvae are differentially attractive to EPNs based on olfactory cues and other unknown factors [
12], we sought to determine how many larvae in a pool were infected by how many EPNs. We found that over time, at an infection rate of 50 IJ/10 μL per larva, EPN numbers increased inside the host from 1 EPN at 1 h to about five EPNs at 6 h (
Figure 4C). We further wondered whether EPNs were more likely to infect
D. melanogaster either through open cavities or through the cuticle. Interestingly, EPNs were more likely to be found in the gut in early infection; however, over time, they were more significantly found within the host hemocoel (
Figure 4D, Χ
2 (12.5, N = 4),
p = 0.0140). This effect may be explained by the loss of tissue integrity at later time points but may indicate a preference at early time points. Since distinct levels of nematode infection varied over time, which affected the rate of bacterial proliferation and thus sepsis for each individual, we then wondered if there was a titer of nematodes that will usually lead to sepsis within 6 h. We found that, when infecting
Drosophila larvae, an effective dose of about 40 IJ/ 10 μL per larva led to at least 50% of the larval population being septic after 6 h (
Figure 4E).
While no change in pH was detected based on bacterial proliferation, though another method may be more sensitive, infected larvae were observed to lose tissue integrity by 6 h of infection. Together, these data indicate that there is an effective dose and time by which EPNs are likely to have overcome the host’s immune system. Also of note, is that the wounds that the EPNs inflict on the host do not cease once it has entered through the gut or the cuticle. Loss of tissue integrity continues throughout the infection, which likely leads to the activation of additional wounding and clotting factors.
3.5. Larval Behavior upon Early Infection
The question arose while observing septic larvae of whether or not they showed signs of lethargy or mortality, due to their lack of movement in later stages. We observed that larvae that were septic were still alive; however, they exhibited lethargy and malaise (
Movie S3). Of note in
Movie S3 is that an EPN can be seen exiting through the mouth during the time-lapse while the larva slowly moves its anterior end towards the left side of the frame. Thus, sickness behavior was observed in septic larvae (
Movie S3) and Smurf larvae (
Figure S2). Those that were infected showed lethargy and decreased gut peristalsis and function, evidenced through the spreading of the dye throughout the larva after several hours as opposed to healthy larvae, which mostly lost gut coloring due to bowel function after the same incubation (
Figure 4). It could be desirable to decrease normal organ function in order to allocate additional resources towards mounting an immune response and overcoming infection [
28].
We then began to wonder how infection may affect other larval behaviors, such as locomotion, at earlier stages of infection. In order to test this, we used an infrared table coupled with FIM tracking software designed to follow larvae [
23]. Larvae were infected with Ht-GFP EPNs and then selected for tracking. Representative images of larval tracks showed similar tracks between infected and non-infected larvae (
Figure 5A). The go phase (or a positive score for movement between two stills) and velocity (pixels/frames per second) were both plotted and checked for significance. While larvae did not appear to differ in the amount they moved (Mann–Whitney U = 599.5, n
1 = n
2 = 38,
p = 0.2053 two-tailed), there was a significant difference between control larvae (M = 1.907, S.D. = 1.450) and infected larvae (M = 3.843, S.D. = 1.615) regarding their velocities (t (72) = 5,
p < 0.0001 two-tailed;
Figure 5B,C). Bending and coiling behavior were examined and while coiling (or head to tail touching) did not differ between groups (Mann–Whitney U = 637, n
1 = n
2 = 38,
p = 0.3148 two-tailed;
Figure 5E), larvae that were infected bent themselves much more than control larvae (Χ
2 (33.17, N = 38),
p < 0.0001;
Figure 5D). Further, spine length (or the length of the larva in pixels) was found to be longer in infected larvae (M = 44.86, S.D. = 6.69) as compared to control larvae (M = 33.04, S.D. = 5.47); t (76) = 8.547,
p < 0.0001 (
Figure 5F). Larvae were also analyzed based on their accumulated distance (or the number of pixels they travelled over time) and we found that infected larvae travelled further than control larvae (Mann–Whitney U = 298, n
1 = n
2 = 38,
p < 0.0001, two-tailed;
Figure 5G).
Thus, larvae in early infection scenarios showed significantly different behaviors in terms of velocity, bending, and distance travelled but not for movement, coiling, or spine length. While control and infected larvae may have moved equally in the go phase, infected larval tracks appeared to be more zigzagged, which could correspond with their increased bending and spine length. Rather than showing sickness behavior, as observed in septic larvae, the early behavioral response to infection seemed to lead to greater energy expenditure. Perhaps the behavior from early infected larvae demonstrates the individual’s last-ditch effort to rid itself of infection, or perhaps it helped the larva initiate a strong immune defense—further research is necessary to elucidate whether or not there are any advantages or disadvantages conferred by these behaviors.
4. Discussion
Nematodes and their symbiotic bacteria represent a complex infection system. In our study, we focused on the early infection wounding, entry, and proliferation events that have been known but have evaded inspection at this level of high resolution and detail. We found that the EPN, Heterorhabditis bacteriophora, enters through the cuticle with a drill-like motion, twisting around its focal anchorage point to dislodge the inner cavity from the outer sheath. While this process has been observed to occur at higher frequencies under a 1-h, in our scenario, the EPN entered after about 1.5 h. This may perhaps indicate the importance of the EPN having a definitive surface to push off from to enter the host. The sheath subsequently stayed attached to the outside while the EPN started to regurgitate bacteria within the first hour of entry. After about 6 h, the symbiotic bacteria, Photorhabdus luminescens, was detected throughout the entire cavity of the larva. Unsurprisingly, the speed of bacterial proliferation increased with higher numbers of EPNs, leading more rapidly to septicemia, likely aided by the open circulatory system of the larva and combined inoculation from multiple nematodes. Subsequently, signs of swelling or edema were observed. Finally, EPNs were observed to freely exit and on occasion re-enter the host through the mouth after infection had occurred.
EPNs were observed to selectively swarm a host prior to infection compared to nearby larvae that were free of EPNs. A swarming behavior may be evolutionarily advantageous for taking down a single individual host but may also reflect unknown olfactory or fitness cues that make a single host more attractive than another [
12]. After wounding the larva and gaining entry, frequently through the gut in earlier time points while through the cuticle in later time points, septicemia occurred in early stages of infection regardless of the number of nematodes that entered. While septic, larvae remained alive and continued to fight the infection; thereafter, they were overwhelmed by tissue degradation and virulence factors, eventually leading to mortality. While it is unknown if any host behavior is directly beneficial to the EPN while inside, it is possible that the behavior of an infected individual likely sends cues to surrounding larvae about the danger of pathogenic threats in the area [
29,
30]. Furthermore, infected larvae exhibited traits of known sickness behaviors, which included malaise, lethargy, and reduced bowel movements, as evidenced through the dye maintained in their guts for several hours longer than the control. Such sickness behavior presumably shifts metabolic costs from motility and feeding to an energetically intensive immune response [
28,
31,
32].
Through the use of the glue method for larval immobilization in conjunction with high-resolution microscopy, we developed a method for gaining a clear and accurate understanding of the early infection kinetics of EPNs. These combined methods can help bring clarity to the host’s immune response as well. With the array of genetics tools available to
Drosophila, many reporter lines and fluorescently tagged proteins can be followed in real-time in response to EPN infection or other infectious agents. Furthermore, several factors have been identified in the
Drosophila host response against nematodes [
13,
15]. Testing, for example, the rate of bacterial proliferation and success of infection using knockdowns and mutant strains will be of great interest for the field.
Furthering our understanding of this complex tripartite system is of particular importance in the context of wound healing. Wound- and clot-associated proteins have been reported to have pleiotropic effects regarding traditional wound healing, protection against nematode entry, as well as an immunological function against the nematode once the EPN has breached the barrier [
15], possibly through the dual roles of extracellular matrix components and crosslinking proteins, which act in conjunction to inhibit the EPN’s entry. Other clotting and immune genes, which include
IDGF3 and the complement-like
TEP3 genes, have an anti-nematode role, which may make EPN infection akin to a murine type-II immune response [
14,
33,
34]. One clotting gene that is most conserved across all organisms is transglutaminase, or factor XIIIa in humans, and has been found to have an anti-nematode effect in
Drosophila [
16]. In response, EPNs have developed several strategies to overcome immunity and have been reported to secrete peptidases and inhibitors that have specific activity against prophenoloxidases and other clotting genes [
35,
36]. Other nematode species have developed a protective mechanism against reactive oxygen species from the host [
37]. Interestingly, nematodes require transglutaminase to catalyze reactions for development, body maintenance, and morphology [
38]. Whether or not transglutaminase is needed to invade the host and bind to the cuticular barrier is currently unknown. This evolutionary arms race is expected according to the red queen hypothesis, which posits that hosts and parasites evolve more distinct and specific responses towards one another, ultimately driving their coevolution [
2,
39]. Thus, with such a specific species interaction, EPNs present themselves as a model system in
Drosophila for furthering our understanding of host–parasite interactions. Not only can this system help us to learn more about nematode infections, but it can also bring forth greater clarity concerning wounding, edema, septicemia, and other advanced inflammatory responses [
40].