Evaluation of d-Limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp.
Abstract
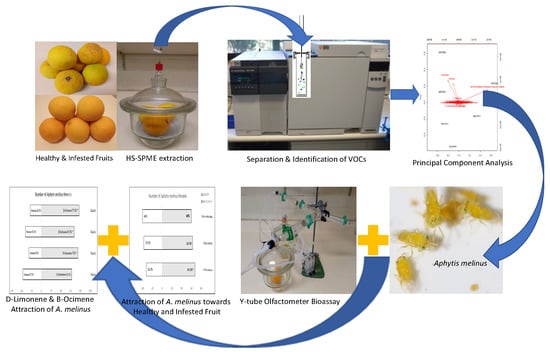
:1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Plant Material
2.3. Extraction of VOCs and GC–MS Analysis
2.4. Y-Tube Olfactometer Behavioral Experiments
2.5. Statistical Analysis
3. Results
3.1. Identification of VOCs
3.2. Behavioural Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray–Darling Basin Authority. Guide to the Proposed Basin Plan; Murray–Darling Basin Authority: Canberra, Australia, 2010. [Google Scholar]
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Açai to Citrus; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 437–516. [Google Scholar] [CrossRef]
- Tena, A.; Garcia-Marí, F. Current situation of citrus pests and diseases in the Mediterranean basin. IOBC Bull. 2011, 62, 365–368. [Google Scholar]
- Habib, A.; Salama, H.S.; Amin, A.H. Population of Aonidiella aurantii on citrus varieties in relation to their physical and chemical characteristics. Entomol. Exp. Appl. 1972, 15, 324–328. [Google Scholar] [CrossRef]
- Beardsley, J.W., Jr.; Gonzalez, R.H. The biology and ecology of armored scales. Ann. Rev. Entomol. 1975, 20, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.D.; Yu, D.S.; Luck, R.F. Variation in life history parameters of California red scale on different citrus cultivars. Ecology 1990, 71, 1451–1460. [Google Scholar] [CrossRef]
- Vacas, S.; Alfaro, C.; Navarro-Llopis, V.; Primo, J. Mating disruption of California red scale, Aonidiella aurantii Maskell (Homoptera: Diaspididae), using biodegradable mesoporous pheromone dispensers. Pest Manag. Sci. 2010, 66, 745–751. [Google Scholar] [CrossRef]
- Mellado, J.J.S. Biological Control of California Red Scale, Aonidiella aurantii (Hemiptera: Diaspididae): Spatial and Temporal Distribution of Natural Enemies, Parasitism Levels and Climate Effects. Ph.D. Thesis, Polytechnic University of Valencia, Valencia, Spain, 2012. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef] [Green Version]
- Paré, P.W.; Farag, M.A. Natural enemy attraction to plant volatiles. In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2004; pp. 1534–1535. [Google Scholar] [CrossRef]
- Benelli, G.; Revadi, S.; Carpita, A.; Giunti, G.; Raspi, A.; Anfora, G.; Canale, A. Behavioral and electrophysiological responses of the parasitic wasp Psyttalia concolor (Szépligeti)(Hymenoptera: Braconidae) to Ceratitis capitata-induced fruit volatiles. Biol. Control 2013, 64, 116–124. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.A.; Tiznado-Hernandez, M.E.; Zavaleta-Gatica, R.; Martınez-Téllez, M.A. Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem. Biophys. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Ann. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Kaplan, I. Trophic complexity and the adaptive value of damage-induced plant volatiles. PLoS Biol. 2012, 10, e1001437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, K.; Agarwal, M.; Mewman, J.; Ren, Y. Optimization and Validation for Determination of VOCs from Lime Fruit Citrus aurantifolia (Christm.) with and without California Red Scale Aonidiella aurantii (Maskell) Infested by Using HS-SPME-GC-FID/MS. World Acad. Sci. Eng. Technol. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2017, 9, 771–775. [Google Scholar]
- Rizqi, A.; Bouchakour, M.; Aberbach, A.; Nia, M. The use of Aphytis melinus for control of California Red Scale in Citrus growing region of Souss in Morocco. In Proceedings of the 10th International Citrus Congress, III, Agadir, Morocco, 15–20 February 2004; Volume 991. [Google Scholar]
- Tena, A.; Urbaneja, A. Feeding behaviour of a California red scale parasitoid in citrus orchards. Acta Hortic. 2015, 1065, 1145–1148. [Google Scholar] [CrossRef]
- Morgan, D.J.; Hare, J.D. Volatile cues used by the parasitoid, Aphytis melinus, for host location: California red scale revisited. Entomol. Exp. Appl. 1998, 88, 235–245. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Du, X.B.; Newman, J.; Ren, Y. Behavioural responses of the parasitoid Aphytis melinus to volatiles organic compounds (VOCs) from Aonidiella aurantii on its host fruit Tahitian lime fruit Citrus latifolia. Biol. Control 2019, 133, 103–109. [Google Scholar] [CrossRef]
- Sternlicht, M. Parasitic wasps attracted by the sex pheromone of their coccid host. Entomophaga 1973, 18, 339–342. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Newman, J.; Ren, Y. Optimization of headspace solid-phase microextraction conditions for the identification of volatiles compounds from the whole fruit of lemon, lime, mandarin and orange. J. Biosci. Med. 2017, 5, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Auta, M.; Musa, U.; Tsado, D.G.; Faruq, A.A.; Isah, A.G.; Raji, S.; Nwanisobi, C. Optimization of citrus peels d-limonene extraction using solvent-free microwave green technology. Chem. Eng. Commun. 2018, 205, 789–796. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Zhang, Z.F.; Tian, H.G.; Liu, T.X. Volatile β-ocimene can regulate developmental performance of peach aphid Myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef]
- Carrasco, M.; Montoya, P.; Cruz-Lopez, L.; Rojas, J.C. Response of the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) to mango fruit volatiles. Environ. Entomol. 2005, 34, 576–583. [Google Scholar] [CrossRef]
- Pinto-Zevallos, D.M.; Hellén, H.; Hakola, H.; van Nouhuys, S.; Holopainen, J.K. Induced defences of Veronica spicata: Variability in herbivore-induced volatile organic compounds. Phytochem. Lett. 2013, 6, 653–656. [Google Scholar] [CrossRef]
- Canale, A. Psyttalia concolor (Hymenoptera Braconidae): Role of host movement and host substrate in ovipositor-probing behaviour. Bull. Insectol. 2003, 56, 211–214. [Google Scholar]
- Zimba, K.; Hill, M.P.; Moore, S.D.; Heshula, U. Agathis bishopi (Hymenoptera: Braconidae) as a potential tool for detecting oranges infested with Thaumatotibia leucotreta (Lepidoptera: Tortricidae). J. Insect Behav. 2015, 28, 618–633. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Flamini, G.; Michaud, J.P.; Canale, A. Innate and learned responses of the tephritid parasitoid Psyttalia concolor (Hymenoptera: Braconidae) to olive volatiles induced by Bactrocera oleae (Diptera: Tephritidae) infestation. J. Econ. Entomol. 2016, 109, 2272–2280. [Google Scholar] [CrossRef]
- Uefune, M.; Choh, Y.; Abe, J.; Shiojiri, K.; Sano, K.; Takabayashi, J. Application of synthetic herbivore-induced plant volatiles causes increased parasitism of herbivores in the field. J. Appl. Entomol. 2012, 136, 561–567. [Google Scholar] [CrossRef]
- Clavijo Mccormick, A.N.D.R.E.A.; Gershenzon, J.; Unsicker, S.B. Little peaks with big effects: Establishing the role of minor plant volatiles in plant–insect interactions. Plant Cell Environ. 2014, 37, 1836–1844. [Google Scholar] [CrossRef] [Green Version]
- Zimba, K.J. Using the Larval Parasitoid, Agathis Bishopi (Nixon) (Hymenoptera: Braconidae), for Early Detection of False Codling Moth, Thaumatotibia Leucotreta (Meyrick) (Lepidoptera: Tortricidae) Infested Fruit. Ph.D. Thesis, Rhodes University, Grahamstown, South Afica, 2014. [Google Scholar]
- Cardé, R.T.; Willis, M.A. Navigational strategies used by insects to find distant, wind-borne sources of odour. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef]



| Feature ID | NIST RI | Chemical Compounds | Lemon | Mandarin | Orange | |||
|---|---|---|---|---|---|---|---|---|
| Non-Infested | Infested | Non-Infested | Infested | Non-Infested | Infested | |||
| 4.464–151.1 | 1302 * | Methoxyphenyl oxime | 4.235 ± 1.382 | 4.749 ± 0.370 | 5.568 ± 1.504 | 4.512 ± 1.337 | 12.081 ± 2.677 | 9.811 ± 0.433 |
| 4.924–136.1 | 966 | β-Thujene | n.d | n.d | 0.731 ± 0.050 | 2.686 ± 1.127 | n.d | n.d |
| 5.921–136.1 | 979 | β-Pinene | 4.464 ± 0.064 | 5.269 ± 2.531 | n.d | n.d | n.d | n.d |
| 6.171–136.1 | 991 | β-Myrcene | 3.359 ± 2.009 | 6.115 ± 2.429 | 1.211 ± 0.405 | 6.752 ± 3.215 | 0.984 ± 0.571 | 1.636 ± 0.918 |
| 6.594–144.1 | 1011 | 3-Carene | n.d | n.d | n.d | n.d | n.d | 2.754 ± 0.4046 * |
| 7.043–136.1 | 1018 | d-Limonene | 30.852 ± 17.394 | 136.594 ± 18.874 * | 31.585 ± 6.825 | 137.129 ± 30.916 * | 24.716 ± 1.878 | 40.918 ± 2.773 * |
| 7.429–136.1 | 1037 | β-Ocimene | 67.916 ± 6.775 | 169.708 ± 13.934 * | 45.039 ± 18.482 | 120.268 ± 10.677 * | 20.812 ± 3.123 | 83.942 ± 15.674 * |
| 7.625–136.1 | 1047 | γ-Terpinene | 3.686 ± 2.522 | 12.214 ± 7.216 | 7.117 ± 2.606 | 41.078 ± 11.193 * | 0.573 ± 0.498 | 1.633 ± 0.929 |
| 8.258–136.1 | 1088 | Terpinolene | 2.449 ± 1.443 | 1.988 ± 1.018 | 1.431 ± 0.251 | 5.572 ± 2.170 | n.d | n.d |
| 8.808–150.1 | 1116 | (3E)-4,8-Dimethyl-1,3,7-nonatriene | 35.130 ± 15.029 | 18.611 ± 1.789 | 17.214 ± 7.774 | 31.584 ± 16.498 | 3.774 ± 1.832 | 32.767 ± 10.184 * |
| 9.092–134.1 | 1131 | Cosmene | 4.025 ± 0.683 | 6.200 ± 0.870 | 0.635 ± 0.319 | 1.238 ± 0.622 | 1.561 ± 0.586 | 3.577 ± 1.367 |
| 9.338–136.1 | 1144 | Alloocimene | 3.653 ± 2.236 | 5.718 ± 2.126 | 1.108 ± 0.198 | 6.906 ± 0.021 * | n.d | 2.409 ± 0.641 * |
| 9.867–128.2 | 1177 | trans-Isopulegone | n.d | n.d | 0.191 ± 0.191 | 0.218 ± 0.218 | n.d | n.d |
| 10.086–154.1 | 1182 | 4-Terpineol | n.d | n.d | 0.284 ± 0.146 | 0.896 ± 0.658 | n.d | n.d |
| 10.309–154.1 | 1189 | α-Terpineol | 1.081 ± 0.567 | 1.815 ± 1.334 | 0.525 ± 0.264 | 2.975 ± 2.476 | n.d | 3.004 ± 1.176 * |
| 10.488–170.2 | 1200 | Dodecane | n.d | n.d | 0.785 ± 0.119 | 1.126 ± 0.195 | n.d | n.d |
| 10.614–156.1 | 1206 | Decanal | n.d | n.d | 1.272 ± 0.642 | 1.312 ± 0.667 | 1.416 ± 0.302 | 1.438 ± 0.209 |
| 11.943–152.1 | 1270 | 3,7-Dimethyl-2,6-octadienal | 0.753 ± 0.386 | n.d | n.d | n.d | n.d | n.d |
| 12.478–212.2 | 1275 | 2,6,11-Trimethyldodecane | 1.369 ± 0.819 | 0.745 ± 0.373 | 1.196 ± 0.051 | 1.648 ± 0.335 | 1.371 ± 0.108 | 1.766 ± 0.213 |
| 14.111–200.3 | 1384 | Hexyl caproate | n.d | n.d | n.d | n.d | 4.375 ± 1.713 | 18.305 ± 6.018 * |
| 14.206–204.2 | 1398 | β-Elemene | 7.433 ± 2.337 | 5.899 ± 0.894 | n.d | n.d | 10.546 ± 4.774 | 20.594 ± 5.217 |
| 14.922–204.2 | 1461 | Alloaromadendrene | 17.374 ± 5.366 | 13.587 ± 0.729 | 3.454 ± 0.358 | 7.713 ± 0.894 * | 39.032 ± 7.499 | 119.291 ± 13.459 * |
| 15.236–204.2 | 1490 | α-Bulnesene | 1.309 ± 0.805 | n.d | n.d | n.d | n.d | n.d |
| 15.384–204.2 | 1440 | Aromandendrene | n.d | n.d | n.d | n.d | 4.920 ± 1.749 | 9.635 ± 2.296 |
| 15.784–204.2 | 1469 | 5,4-di-epi-Aristolochene | 2.391 ± 0.345 * | 1.435 ± 0.103 | n.d | n.d | 3.789 ± 1.474 | 7.687 ± 0.861 |
| 15.941–204.2 | 1527 | Panasinsene | 16.519 ± 6.276 | 10.936 ± 2.061 | n.d | n.d | 11.113 ± 4.743 | 20.255 ± 2.813 |
| 16.283–204.2 | 1544 | Eudesma-4(14),7(11)-diene | 51.037 ± 45.977 | 101.571 ± 76.637 | n.d | n.d | 100.882 ± 27.236 | 113.043 ± 38.383 |
| 17.039–204.2 | 1556 | Guaia-3,9-diene | 1.705 ± 0.296 | 2.091 ± 0.595 | n.d | n.d | n.d | n.d |
| 17.323–222.2 | 1564 | Nerolidol | 3.129 ± 2.278 | 2.652 ± 1.351 | n.d | n.d | n.d | 1.247 ± 0.644 |
| 17.532–218.2 | 1581 | (3E,7E)-4,8,12-Trimethyltrideca-1,3,7,11- tetraene | n.d | n.d | 1.941 ± 0.766 | 2.587 ± 1.083 | n.d | 1.582 ± 0.821 * |
| 17.849–220.2 | 1586 | trans-(Z)-α-Bisabolene epoxide | 1.355 ± 0.319 | 1.322 ± 0.359 | 0.142 ± 0.1416 | 0.155 ± 0.155 | n.d | n.d |
| 18.945–222.2 | 1660 | Neointermedeol | 3.218 ± 0.332 | 4.672 ± 0.699 | 0.348 ± 0.182 | 0.566 ± 0.288 | 1.275 ± 0.332 | 2.305±.389 |
| 23.368–256.2 | 1968 | n-Hexadecanoic acid | n.d | 0.920 ± 0.920 | 3.098 ± 1.076 | 3.336 ± 0.928 | n.d | n.d |
| 44.331–722.6 | 4932 * | Trimyristin | 2.843 ± 2.201 | 2.088 ± 0.529 | 1.343 ± 0.458 | 1.416 ± 0.266 | n.d | n.d |
| Species | Infested Citrus Fruit | Non-Infested Citrus Fruit | F | p-Value | ||
|---|---|---|---|---|---|---|
| Choice Time (s) Mean ± SE | Replicates | Choice Time (s) Mean ± SE | Replicates | |||
| Lemon | 148.15 ± 13.482 | 27 | 133.31 ± 14.604 | 13 | 0.558 | 0.206 ns |
| Orange | 153.40 ± 10.523 | 25 | 119.13 ± 12.084 | 15 | 4.573 | 0.140 ns |
| Mandarin | 139.53 ± 12.802 | 23 | 144.06 ± 15.412 | 17 | 0.051 | 0.194 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of d-Limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. https://doi.org/10.3390/insects11010044
Mohammed K, Agarwal M, Li B, Newman J, Liu T, Ren Y. Evaluation of d-Limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects. 2020; 11(1):44. https://doi.org/10.3390/insects11010044
Chicago/Turabian StyleMohammed, Khalid, Manjree Agarwal, Beibei Li, James Newman, Tao Liu, and Yonglin Ren. 2020. "Evaluation of d-Limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp." Insects 11, no. 1: 44. https://doi.org/10.3390/insects11010044