Low Host Specialization in the Cuckoo Wasp, Parnopes grandior, Weakens Chemical Mimicry but Does Not Lead to Local Adaption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Behavioural Experiments
2.3. Characterization of the Cuticular Hydrocarbon Profile (CHC)
2.4. Statistical Analysis
3. Results
3.1. Behavioural Interactions
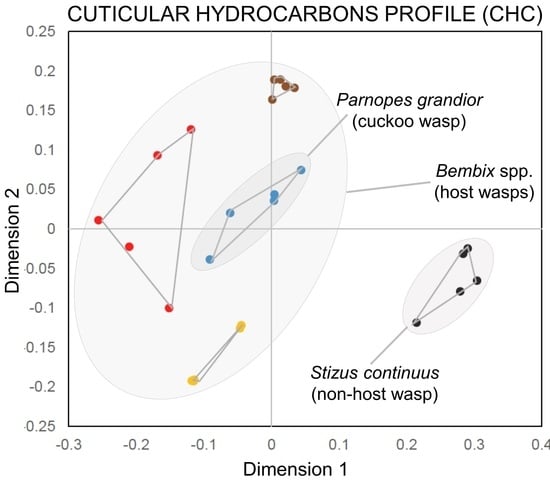
3.2. Characterization of CHC Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Substance | RI | B. sinuata | B. zonata | B. merceti | P. grandior | S. continuus |
|---|---|---|---|---|---|---|
| C20 | 2000 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.00 |
| C21en1 | 2073 | 0.00 | 0.24 ± 0.19 | 0.00 | 0.01 ± 0.01 | 0.00 |
| C21en2 | 2079 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.00 | 0.00 | 0.00 |
| C21 | 2100 | 0.07 ± 0.02 | 1.73 ± 0.94 | 0.04 ± 0.01 | 0.25 ± 0.10 | 0.00 |
| C22en1 | 2172 | 0.00 | 0.05 ± 0.04 | 0.02 ± 0.02 | 0.00 | 0.00 |
| C22 | 2200 | 0.06 ± 0.04 | 0.07 ± 0.03 | 0.05 ± 0.00 | 0.06 ± 0.01 | 0.00 |
| C23en1 | 2271 | 0.76 ± 0.10 | 0.60 ± 0.44 | 0.07 ± 0.02 | 0.00 | 0.00 |
| C23en2 | 2275 | 0.00 | 1.87 ± 1.77 | 0.02 ± 0.01 | 0.10 ± 0.04 | 0.00 |
| C23en3 | 2278 | 0.13 ± 0.04 | 0.14 ± 0.04 | 0.04 ± 0.01 | 0.10 ± 0.05 | 0.00 |
| C23en5 | 2292 | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.00 | 0.00 |
| C23 | 2300 | 11.66 ± 0.40 | 11.68 ± 1.74 | 3.11 ± 0.22 | 1.54 ± 0.41 | 0.28 ± 0.04 |
| 11,9mC23 | 2333 | 0.04 ± 0.03 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| 5mC23 | 2348 | 0.02 ± 0.01 | 0.00 | 0.14 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| 3mC23/C24en1 | 2373 | 0.74 ± 0.06 | 0.00 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| C24 | 2400 | 0.58 ± 0.02 | 0.32 ± 0.12 | 0.18 ± 0.01 | 0.12 ± 0.02 | 0.08 ± 0.01 |
| C25dien3 | 2458 | 0.00 | 0.00 | 0.03 ± 0.01 | 0.00 | 0.00 |
| C25dien4 | 2463 | 0.00 | 0.00 | 0.05 ± 0.01 | 0.00 | 0.00 |
| C25en1 | 2469 | 0.00 | 1.21 ± 0.38 | 1.18 ± 0.36 | 0.05 ± 0.05 | 0.00 |
| C25en2 | 2473 | 0.00 | 0.73 ± 0.73 | 0.00 | 0.11 ± 0.04 | 0.13 ± 0.05 |
| C25en3 | 2477 | 27.17 ± 1.38 | 0.26 ± 0.08 | 0.38 ± 0.10 | 0.00 | 0.00 |
| C25en4 | 2481 | 0.00 | 0.37 ± 0.15 | 0.93 ± 0.11 | 0.19 ± 0.07 | 0.00 |
| C25en5 | 2485 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.01 |
| C25en6 | 2492 | 0.00 | 0.00 | 0.65 ± 0.09 | 0.00 | 0.00 |
| C25 | 2500 | 12.70 ± 0.42 | 10.45 ± 1.33 | 5.57 ± 0.31 | 2.49 ± 0.32 | 2.08 ± 0.55 |
| 13,11,9mC25 | 2531 | 0.07 ± 0.05 | 0.03 ± 0.01 | 0.71 ± 0.08 | 0.03 ± 0.01 | 0.40 ± 0.16 |
| 7mC25 | 2538 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.01 | 0.21 ± 0.11 |
| 5mC25 | 2549 | 0.00 | 0.01 ± 0.01 | 0.30 ± 0.03 | 0.28 ± 0.08 | 0.19 ± 0.06 |
| 3mC25 / C26en2 | 2574 | 1.07 ± 0.10 | 0.07 ± 0.02 | 0.12 ± 0.02 | 0.06 ± 0.03 | 0.91 ± 0.33 |
| C26en3 | 2582 | 0.00 | 0.01 ± 0.01 | 0.00 | 0.08 ± 0.03 | 0.00 |
| 5,15dimC25/C26en3 | 2582 | 0.00 | 0.00 | 0.27 ± 0.02 | 0.00 | 0.00 |
| 5,9/5,11/5,13/5,15dimC25 | 2585 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 ± 0.08 |
| C26en4 | 2593 | 0.00 | 0.00 | 0.05 ± 0.01 | 0.00 | 0.00 |
| C26 | 2600 | 0.29 ± 0.02 | 0.54 ± 0.07 | 0.89 ± 0.03 | 0.81 ± 0.13 | 0.66 ± 0.11 |
| 3,7dimC25 | 2612 | 0.00 | 0.00 | 0.00 | 0.00 | 0.26 ± 0.11 |
| 13,12,11,10,9mC26 | 2632 | 0.00 | 0.00 | 0.00 | 0.00 | 0.37 ± 0.10 |
| 8,10dimC26 | 2641 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 ± 0.09 |
| 6mC26 | 2652 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.01 |
| C27dien1 | 2653 | 0.00 | 0.06 ± 0.04 | 0.14 ± 0.01 | 0.00 | 0.00 |
| 5mC26 | 2657 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 ± 0.02 |
| C27dien | 2660 | 0.00 | 0.01 ± 0.01 | 0.24 ± 0.02 | 0.00 | 0.00 |
| 4mC26 | 2663 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 ± 0.01 |
| C27en1 | 2665 | 0.00 | 0.82 ± 0.30 | 0.47 ± 0.04 | 0.00 | 0.00 |
| C27en2 | 2669 | 0.00 | 0.30 ± 0.19 | 0.77 ± 0.25 | 1.04 ± 0.41 | 0.00 |
| C27en3 | 2672 | 0.00 | 0.00 | 0.93 ± 0.37 | 0.19 ± 0.08 | 2.11 ± 0.70 |
| C27en4 | 2676 | 14.39 ± 1.09 | 0.76 ± 0.31 | 1.02 ± 0.05 | 0.72 ± 0.44 | 0.00 |
| C27en5 | 2680 | 0.00 | 0.34 ± 0.11 | 4.23 ± 0.42 | 1.85 ± 0.67 | 0.00 |
| C27en6 | 2691 | 0.00 | 0.00 | 2.61 ± 0.29 | 0.00 | 0.11 ± 0.07 |
| 4,8/4,10/4,12dimC26 | 2693 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 ± 0.02 |
| C27 | 2700 | 4.54 ± 0.27 | 18.52 ± 2.89 | 29.35 ± 0.97 | 22.53 ± 1.89 | 14.72 ± 2.34 |
| 13,11,9mC27 | 2731 | 0.06 0.04 | 0.02 ± 0.02 | 0.87 ± 0.10 | 0.31 ± 0.07 | 9.97 ± 1.69 |
| 7mC27 | 2737 | 0.00 | 0.00 | 0.01 ± 0.01 | 0.03 ± 0.02 | 1.86 ± 0.31 |
| 5mC27 | 2748 | 0.00 | 0.02 ± 0.02 | 0.29 ± 0.05 | 0.08 ± 0.03 | 2.70 ± 0.62 |
| 9,13/9,15/9,17dimC27 | 2764 | 0.00 | 0.00 | 0.00 | 0.00 | 0.16 ± 0.07 |
| C28en1 | 2766 | 0.00 | 0.09 ± 0.03 | 0.01 ± 0.01 | 0.00 | 0.00 |
| 3mC27/C28en2 | 2773 | 0.28 ± 0.05 | 0.25 ± 0.05 | 0.22 ± 0.03 | 1.63 ± 0.17 | 11.31 ± 0.97 |
| 5,17dimC27/C28en3 | 2779 | 0.00 | 0.00 | 0.23 ± 0.03 | 0.00 | 0.00 |
| 5,15dimC27 | 2783 | 0.00 | 0.00 | 0.00 | 0.00 | 3.39 ± 0.87 |
| C28en4 | 2794 | 0.00 | 0.00 | 0.09 ± 0.01 | 0.00 | 0.00 |
| C28 | 2800 | 0.62 ± 0.03 | 0.82 ± 0.08 | 1.19 ± 0.02 | 0.89 ± 0.19 | 1.78 ± 0.25 |
| 3,15dimC27 | 2807 | 0.00 | 0.00 | 0.00 | 0.00 | 0.58 ± 0.24 |
| 3,7dimC27 | 2810 | 0.00 | 0.00 | 0.00 | 0.00 | 1.35 ± 0.27 |
| 14,13,12,11,10mC28 | 2829 | 0.00 | 0.00 | 0.00 | 0.00 | 0.92 ± 0.30 |
| 8,12/8,14/8,16dimC28 | 2838 | 0.00 | 0.00 | 0.00 | 0.00 | 0.79 ± 0.25 |
| 6mC28 | 2847 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 ± 0.02 |
| C29dien2 | 2849 | 0.00 | 0.25 ± 0.18 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.00 |
| 5mC28 | 2853 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 ± 0.03 |
| C29dien3 | 2855 | 0.00 | 0.11 ± 0.09 | 0.27 ± 0.04 | 0.09 ± 0.05 | 0.00 |
| 4mC28 | 2861 | 0.00 | 0.00 | 0.00 | 0.00 | 0.28 ± 0.08 |
| C29dien4 | 2865 | 0.00 | 0.00 | 1.33 ± 0.09 | 0.29 ± 0.12 | 0.00 |
| C29en2 | 2866 | 0.00 | 3.88 ± 1.28 | 0.00 | 0.00 | 0.00 |
| 3mC28/C29en4 | 2875 | 3.87 ± 0.46 | 3.29 ± 1.09 | 2.96 ± 0.23 | 36.72 ± 2.40 | 2.35 ± 0.39 |
| C29en5 | 2878 | 0.00 | 0.46 ± 0.29 | 1.27 ± 0.09 | 0.00 | 0.00 |
| C29en6 | 2882 | 0.00 | 0.34 ± 0.24 | 1.78 ± 0.24 | 3.39 ± 0.98 | 0.00 |
| C29en7 | 2893 | 0.00 | 0.00 | 2.93 ± 0.37 | 0.00 | 0.00 |
| C29 | 2900 | 12.57 ± 0.83 | 16.97 ± 1.74 | 23.97 ± 0.79 | 12.91 ± 2.68 | 13.12 ± 1.06 |
| 15,13,11,9mC29 | 2929 | 0.11 ± 0.07 | 0.00 | 0.35 ± 0.05 | 0.13 ± 0.05 | 9.01 ± 3.68 |
| 7mC29 | 2945 | 0.00 | 0.00 | 0.00 | 0.00 | 1.07 ± 0.25 |
| 5mC29 | 2947 | 0.00 | 0.03 ± 0.03 | 0.48 ± 0.07 | 0.04 ± 0.03 | 2.33 ± 0.44 |
| 7,11/7,15/7,17dimC29 | 2969 | 0.00 | 0.00 | 0.00 | 0.00 | 0.62 ± 0.25 |
| 3mC29/C30en1 | 2973 | 0.00 | 0.30 ± 0.19 | 0.10 ± 0.02 | 0.99 ±10 | 3.97 ± 2.04 |
| C30en2 | 2976 | 0.14 ± 0.04 | 0.13 ± 0.08 | 0.00 | 0.00 | 0.00 |
| 5,17dimC29/C30en3 | 2977 | 0.00 | 0.00 | 0.09 ± 0.03 | 0.00 | 0.00 |
| 5,13/5,15/5,17dimC29 | 2982 | 0.00 | 0.00 | 0.00 | 0.00 | 2.30 ± 0.80 |
| C30 | 3000 | 0.57 ± 0.05 | 0.41 ± 0.04 | 0.40 ± 0.04 | 0.13 ± 0.04 | 1.05 ± 0.16 |
| 3,7dimC29 | 3020 | 0.00 | 0.00 | 0.00 | 0.00 | 0.29 ± 0.10 |
| 15,14,13mC30 | 3030 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 ± 0.04 |
| 6mC30 | 3046 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 ± 0.03 |
| C31dien2 | 3047 | 0.00 | 0.98 ± 0.54 | 0.00 | 0.00 | 0.00 |
| C31dien3 | 3052 | 0.00 | 0.65 ± 0.53 | 0.05 ± 0.02 | 0.01 ± 0.01 | 0.00 |
| C31dien4 | 3060 | 0.00 | 0.00 | 0.06 ± 0.03 | 0.04 ± 0.04 | 0.00 |
| C31en2 | 3069 | 0.00 | 8.26 ± 2.55 | 0.04 ± 0.04 | 0.71 ± 0.15 | 0.00 |
| C31en3 | 3077 | 0.86 ± 0.11 | 1.96 ± 1.29 | 0.71 ± 0.05 | 6.72 ± 1.39 | 0.39 ± 0.24 |
| C31en4 | 3085 | 0.00 | 0.00 | 0.28 ± 0.02 | 0.11 ± 0.11 | 0.00 |
| C31 | 3100 | 5.89 ± 0.76 | 4.03 ± 1.04 | 5.04 ± 0.42 | 1.35 ± 0.36 | 1.95 ± 0.37 |
| 15,13,11mC31 | 3228 | 0.12 ± 0.08 | 0.00 | 0.01 ± 0.01 | 0.07 ± 0.03 | 1.59 ± 0.91 |
| 7mC31 | 3142 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 ± 0.03 |
| 5mC31 | 3147 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.24 ± 0.07 |
| C32en1 | 3165 | 0.00 | 0.27 ± 0.09 | 0.00 | 0.00 | 0.00 |
| 3mC31/C32en2 | 3172 | 0.00 | 0.00 | 0.01 ± 0.01 | 0.00 | 0.50 ± 0.13 |
| C32 | 3200 | 0.11 ± 0.02 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.11 ± 0.03 |
| C33dien2 | 3244 | 0.00 | 1.44 ± 0.92 | 0.00 | 0.00 | 0.00 |
| C33en2 | 3266 | 0.00 | 2.04 ± 0.53 | 0.00 | 0.56 ± 0.18 | 0.00 |
| C33 | 3300 | 0.48 ± 0.08 | 0.52 ± 0.22 | 0.13 ± 0.03 | 0.09 ± 0.01 | 0.14 ± 0.07 |
| 17,15,13,11,9,7mC33 | 3322 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 ± 0.06 |
| C34en1 | 3364 | 0.00 | 0.63 ± 0.34 | 0.00 | 0.00 | 0.00 |
| C35 | 3500 | 0.00 | 0.57 ± 0.19 | 0.00 | 0.00 | 0.00 |
References
- Rosenheim, J.A. Parasite presence acts as a proximate cue in the nest-site selection process of the solitary digger wasp, Ammophila dysmica (Hymenoptera: Sphecidae). J. Insect Behav. 1988, 1, 333–342. [Google Scholar] [CrossRef]
- Ballesteros, Y.; Tormos, J.; Gayubo, S.F.; Asís, J.D. Notes on the prey, nesting behaviour and natural enemies of three Bembix sand wasps (Hymenoptera: Crabronidae) in the Iberian Peninsula. Annales de la Société Entomologique de France 2012, 48, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Polidori, C.; Borruso, L.; Boesi, R.; Andrietti, F. Segregation of temporal and spatial distribution between kleptoparasites and parasitoids of the eusocial sweat bee, Lasioglossum malachurum (Hymenoptera: Halictidae, Mutillidae). Entomol. Sci. 2009, 12, 116–129. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998; p. 392. [Google Scholar]
- Poulin, R.; Morand, S.; Skorping, A. Evolutionary Biology of Host–Parasite Relationships: Theory Meets Reality; Elsevier: Amsterdam, Netherlands, 2000; p. 260. [Google Scholar]
- Lenoir, A.; d’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.M. Solitary Wasps: Natural History and Behavior; Cornell University Press: Ithaca, NY, USA, 2001; p. 424. [Google Scholar]
- Lorenzi, M.C. The result of an arms race: The chemical strategies of Polistes social parasites. Ann. Zool. Fenn. 2006, 43, 550–563. [Google Scholar]
- Michener, C.D. The Bees of the World; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; p. 992. [Google Scholar]
- Strohm, E.; Kroiss, J.; Herzner, G.; Laurien-Kehnen, C.; Boland, W.; Schreier, P.; Schmitt, T. A cuckoo in wolves’ clothing? Chemical mimicry in a specialized cuckoo wasp of the European beewolf (Hymenoptera, Chrysididae and Crabronidae). Front. Zool. 2008, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Uboni, A.; Bagnères, A.-G.; Christidès, J.P.; Lorenzi, C.M. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 2012, 58, 1259–1264. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.-G. Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; p. 506. [Google Scholar]
- Kreuter, K.; Bunk, E.; Lückemeyer, A.; Twele, R.; Francke, W.; Ayasse, M. How the social parasitic bumblebee Bombus bohemicus sneaks into power of reproduction. Behav. Ecol. Sociobiol. 2012, 66, 475–486. [Google Scholar] [CrossRef]
- Johnson, C.A.; Vander Meer, R.K.; Lavine, B. Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps Emery, after killing a Formica host queen (Hymenoptera: Formicidae). J. Chem. Ecol. 2001, 27, 1787–1804. [Google Scholar] [CrossRef]
- Cini, A.; Bruschini, C.; Signorotti, L.; Pontieri, L.; Turillazzi, S.; Cervo, R. The chemical basis of host nest detection and chemical integration in a cuckoo paper wasp. J. Exp. Biol. 2011, 214, 3698–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurdack, M.; Herbertz, S.; Dowling, D.; Kroiss, J.; Strohm, E.; Baur, H.; Niehuis, O.; Schmitt, T. Striking cuticular hydrocarbon dimorphism in the mason wasp Odynerus spinipes and its possible evolutionary cause (Hymenoptera: Chrysididae, Vespidae). Proc. R. Soc. Lond. B Biol. Sci. 2015, 282, 20151777. [Google Scholar] [CrossRef] [PubMed]
- Kimsey, L.S.; Bohart, R.M. The Chrysidid Wasps of the World; Oxford University Press: New York, NY, USA, 1990; p. 664. [Google Scholar]
- Berland, L.; Bernard, F. Hyménoptères Vespiformes III (Cleptidae, Chrysidae, Trigonalidae) Faune de France vol.: 34; Office Central de Faunistique. Fédération Française des Société des Sciences Naturelles, Le Chevalier: Paris, France, 1938; p. 145. [Google Scholar]
- Grandi, G. Contributi alla conoscenza imenotteri melliferi e predatori. V. Mem. Soc. Entomol. It. 1927, 6, 5–20. [Google Scholar]
- Grandi, G. Studi Di Un Entomologo Sugli Imenotteri Superiori; EdiAgricole: Bologna, Italia, 1961; p. 659. [Google Scholar]
- Linsenmaier, W. Revision der Familie Chrysididae. Zweiter Nachtrag Mitteilungen der Schweizerischen Entomologischen Gesellschaft. 1968, 41, 1–144. [Google Scholar]
- Witt, R. Wespen: Beobachten, Bestimmen; Naturbuch-Verlag: Augsburg, Germany, 1998; p. 359. [Google Scholar]
- Asís, J.D.; Gayubo, S.F.; Tormos, J. Data on the nesting behaviour of five European Bembix and description of the mature larvae of B. merceti and B. rostrata (Hymenoptera, Sphecidae). Deut. Entomol. Zschr. 1992, 39, 221–231. [Google Scholar] [CrossRef]
- Asís, J.D.; Tormos, J.; Gayubo, S.F. Nesting behaviour and provisioning in Bembix merceti and Bembix zonata (Hymenoptera: Crabronidae). J. Nat. Hist. 2004, 38, 1799–1809. [Google Scholar] [CrossRef]
- Evans, H.E.; O’Neill, K.M. The Sand Wasps: Natural History and Behaviour; Harvard University Press: Cambridge, MA, USA, 2007; p. 424. [Google Scholar]
- Saul-Gershenz, L.; Millar, J.G.; McElfresh, J.S.; Williams, N.M. Deceptive signals and behaviors of a cleptoparasitic beetle show local adaptation to different host bee species. Proc. Natl. Acad. Sci. USA 2018, 115, 9756–9760. [Google Scholar] [CrossRef] [Green Version]
- Casacci, L.P.; Schönrogge, K.; Thomas, J.A.; Balletto, E.; Bonelli, S.; Barbero, F. Host specificity pattern and chemical deception in a social parasite of ants. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Torres, C.W.; Tonione, M.A.; Ramírez, S.R.; Sapp, J.R.; Tsutsui, N.D. Genetic and chemical divergence among host races of a socially parasitic ant. Ecol. Evol. 2018, 8, 11385–11398. [Google Scholar] [CrossRef]
- Polidori, C.; Geyer, M.; Schmitt, T. Do Sphecodes cuckoo bees use chemical insignificance to invade the nests of their social Lasioglossum bee hosts? Apidologie 2019, in press. [Google Scholar] [CrossRef]
- Sann, M.; Niehuis, O.; Peters, R.S.; Mayer, C.; Kozlov, A.; Podsiadlowski, L.; Bank, S.; Meusemann, K.; Misof, B.; Bleidorn, C.; et al. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evol. Biol. 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzi, M.C.; Azzani, L.; Bagnères, A.-G. Evolutionary consequences of deception: Complexity and informational content of colony signature are favored by social parasitism. Curr. Zool. 2014, 60, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Ruano, F.; Devers, S.; Sanllorente, O.; Errard, C.; Tinaut, A.; Lenoir, A. A geographical mosaic of coevolution in a slave-making host-parasite system. J. Evol. Biol. 2011, 24, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Polidori, C.; Giordani, I.; Wurdack, M.; Tormos, J.; Asís, J.D.; Schmitt, T. Post-mating shift towards longer-chain cuticular hydrocarbons drastically reduces female attractiveness to males in a digger wasp. J. Insect Physiol. 2017, 100, 119–127. [Google Scholar] [CrossRef]
- Asís, J.D.; Ballesteros, Y.; Tormos, J.; Baños-Picón, L.; Polidori, C. Spatial nest-settlement decisions in digger wasps: Conspecifics matter more than heterospecifics and previous experience. Ethology 2014, 120, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Paxton, R.J.; Kukuk, P.F.; Tengö, J. Effects of familiarity and nestmate number on social interactions in two communal bees, Andrena scotica and Panurgus calcaratus (Hymenoptera, Andrenidae). Insect Soc. 1999, 46, 109–118. [Google Scholar] [CrossRef]
- Palaban, N.; Davey, K.G.; Packer, L. Escalation of aggressive interactions during staged encounters in Halictus ligatus Say (Hymenoptera: Halictidae), with a comparison of circle tube behaviors with other halictine species. J. Insect Behav. 2000, 13, 627–650. [Google Scholar] [CrossRef]
- Boesi, R.; Polidori, C. Nest membership determines the levels of aggression and cooperation between females of a supposedly communal digger wasp. Aggress. Behav. 2011, 37, 405–416. [Google Scholar] [CrossRef]
- Bos, N.; Grinsted, L.; Holman, L. Wax On, Wax Off: Nest Soil Facilitates Indirect Transfer of Recognition Cues between Ant Nestmates. PLoS ONE 2011, 6, e19435. [Google Scholar] [CrossRef]
- Carlson, D.A.; Bernier, U.R.; Sutton, B.D. Elution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 1998, 24, 1845–1865. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Pearson Hall: Upper Saddle River, NJ, USA, 2010; p. 960. [Google Scholar]
- Leyer, I.; Wesche, K. Multivariate Statistik in der Ökologie; Springer: Berlin, Germany, 2007; p. 232. [Google Scholar]
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.; Urban, D. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, ORE, USA, 2002. [Google Scholar]
- Kruskal, J.; Carroll, J.D. Geometrical models and badness-of-fit functions. In Multivariate Analysis; Krishnaiah, P.R., Ed.; Academic Press: New York, NY, USA, 1969; pp. 639–671. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Version 2.15. Paleontol. Eletronica 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 September 2019).
- Turillazzi, S.; Sledge, M.F.; Dani, F.R.; Cervo, R.; Massolo, A.; Fondelli, L. Social hackers: Integration in the host chemical recognition system by a paper wasp social parasite. Naturwissenschaften 2000, 87, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.D.; Menzel, F.; Nehring, V.; Schmitt, T. Ecology and evolution of communication in social insects. Cell 2016, 164, 1277–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akino, T.; Knapp, J.J.; Thomas, J.A.; Elmes, G.W. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 1999, 266, 1419–1426. [Google Scholar] [CrossRef] [Green Version]
- Elmes, G.W.; Barr, B.; Thomas, J.A.; Clarke, R.T. Extreme host specifcity by Microdon mutabilis (Diptera: Syrphidae), a social parasite of ants. Proc. R. Soc. Lond. B 1999, 266, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Polidori, C.; Bevacqua, S.; Andrietti, F. Do digger wasps time their provisioning activity to avoid cuckoo wasps (Hymenoptera: Crabronidae and Chrysididae)? Acta Ethol. 2010, 13, 11–21. [Google Scholar] [CrossRef]
- Brandt, M.; Foitzik, S. Community context and specialization influence the coevolutionary interactions in a slavemaking ant. Ecology 2004, 85, 2997–3009. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Destri, S.; Spencer, S.H.; Turillazzi, S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 2001, 62, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Dani, F.R.; Jones, G.R.; Corsi, S.; Beard, R.; Pradella, D.; Turillazzi, S. Nestmate recognition cues in the honey bee: Differential importance of cuticular alkanes and alkenes. Chem. Senses 2005, 30, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurdack, M.; Polidori, C.; Keller, A.; Feldhaar, H.; Schmitt, T. Release from prey preservation behavior via prey switch allowed diversification of cuticular hydrocarbon profiles in digger wasps. Evolution 2017, 71, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Heinze, J.; Schmitt, T.; Foitzik, S. A chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J. Evol. Biol. 2005, 18, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.R.; Als, T.D.; Maile, R.; Jones, G.R.; Boomsma, J.J. A mosaic of chemical coevolution in a large blue butterfly. Science 2008, 319, 88–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervo, R. Polistes wasps and their social parasites: An overview. Ann. Zool. Fenn. 2006, 43, 531–549. [Google Scholar]
- Dronnet, S.; Simon, X.; Verhaeghe, J.C.; Rasmont, P.; Errard, C. Bumblebee inquilinism in Bombus (Fernaldaepsithyrus) sylvestris (Hymenoptera, Apidae): Behavioural and chemical analyses of host-parasite interactions. Apidologie 2005, 10, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, M.C.; Cervo, R.; Zacchi, F.; Turillazzi, S.; Bagnères, A.-G. Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera Vespidae). Parasitology 2004, 129, 643–651. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; Bagnères, A.G. Concealing identity and mimicking hosts: A dual chemical strategy for a single social parasite? (Polistes atrimandibularis, Hymenoptera: Vespidae). Parasitology 2002, 125, 507–512. [Google Scholar] [CrossRef]
- Martin, S.J.; Takahashi, J.; Ono, M.; Drijfhout, F.P. Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J. Insect Physiol. 2008, 54, 700–707. [Google Scholar] [CrossRef]
- Kroiss, J.; Schmitt, T.; Strohm, E. Low level of cuticular hydrocarbons in a parasitoid of a solitary digger wasp and its potential for concealment. Entomol. Sci. 2009, 12, 9–16. [Google Scholar] [CrossRef]
- Timms, R.; Read, A.F. What makes a specialist special? Trends Ecol. Evol. 1999, 14, 333–334. [Google Scholar] [CrossRef]
- Buschinger, A. Evolution of social parasitism in ants. Trends Ecol. Evol. 1986, 1, 155–160. [Google Scholar] [CrossRef]
- Bauer, S.; Böhm, M.; Witte, V.; Foitzik, S. An ant social parasite in-between two chemical disparate host species. Evol. Ecol. 2010, 24, 317–332. [Google Scholar] [CrossRef]
- von Beeren, C.; Brückner, A.; Maruyama, M.; Burke, G.; Wieschollek, J.; Kronauer, D.J.C. Chemical and behavioral integration of army ant-associated rove beetles—A comparison between specialists and generalists. Front. Zool. 2018, 15, 8. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polidori, C.; Ballesteros, Y.; Wurdack, M.; Asís, J.D.; Tormos, J.; Baños-Picón, L.; Schmitt, T. Low Host Specialization in the Cuckoo Wasp, Parnopes grandior, Weakens Chemical Mimicry but Does Not Lead to Local Adaption. Insects 2020, 11, 136. https://doi.org/10.3390/insects11020136
Polidori C, Ballesteros Y, Wurdack M, Asís JD, Tormos J, Baños-Picón L, Schmitt T. Low Host Specialization in the Cuckoo Wasp, Parnopes grandior, Weakens Chemical Mimicry but Does Not Lead to Local Adaption. Insects. 2020; 11(2):136. https://doi.org/10.3390/insects11020136
Chicago/Turabian StylePolidori, Carlo, Yolanda Ballesteros, Mareike Wurdack, Josep Daniel Asís, José Tormos, Laura Baños-Picón, and Thomas Schmitt. 2020. "Low Host Specialization in the Cuckoo Wasp, Parnopes grandior, Weakens Chemical Mimicry but Does Not Lead to Local Adaption" Insects 11, no. 2: 136. https://doi.org/10.3390/insects11020136