Potential Therapies Targeting the Metabolic Reprogramming of Diabetes-Associated Breast Cancer
Abstract
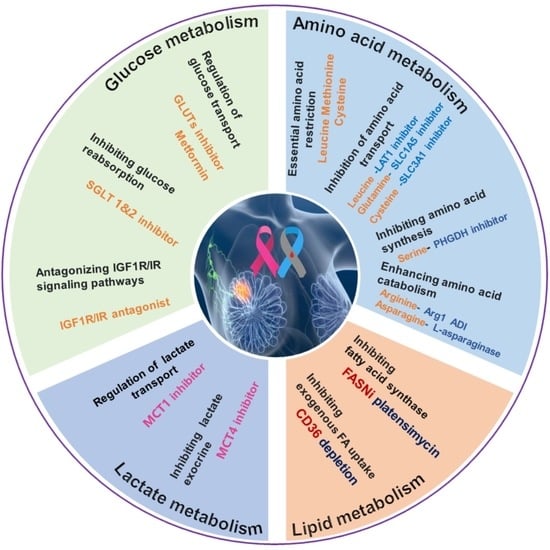
:1. Background
2. MRS Targeting Glucose Metabolism
2.1. Metformin
2.2. GLUT Inhibitor
2.3. SGLT Inhibitor
2.4. MCT Inhibitor
2.5. Insulin Growth Factor Receptor (IGF1R) Antagonist and Insulin Receptor (IR) Antagonist
3. MRS under Amino Acid Metabolism
3.1. Leucine
3.2. Methionine
3.3. Cysteine
3.4. Glutamine and Glutamate
3.5. Arginine
3.6. Serine
3.7. Asparagine
4. MRS Targeting the Lipid Metabolism
4.1. De Novo FA Synthesis
4.2. Exogenous FA Uptake
5. Immune Dysregulation in Diabetes That Maybe Associated with Cancer Progression
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report website. 2022. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 25 November 2022).
- American Cancer Society. Cancer Facts & Figures. 2022, 10. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on 25 November 2022).
- Hardefeldt, P.J.; Edirimanne, S.; Eslick, G.D. Diabetes increases the risk of breast cancer: A meta-analysis. Endocr. Relat. Cancer 2012, 19, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.-B.; Ren, G.-S. Diabetes mellitus and prognosis in women with breast cancer: A systematic review and meta-analysis. Medicine 2016, 95, e5602. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.D.; McGee, S.L. Metabolic reprogramming in type 2 diabetes and the development of breast cancer. J. Endocrinol. 2018, 237, R35–R46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J. mTOR signaling and drug development in cancer. Nat. Rev. Clin. Oncol. 2010, 7, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Tomas, N.M.; Masur, K.; Piecha, J.C.; Niggemann, B.; Zänker, K.S. Akt and phospholipase Cγ are involved in the regulation of growth and migration of MDA-MB-468 breast cancer and SW480 colon cancer cells when cultured with diabetogenic levels of glucose and insulin. BMC Res. Notes 2012, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Faria, J.; Negalha, G.; Azevedo, A.; Martel, F. Metformin and breast cancer: Molecular targets. J. Mammary Gland Biol. Neoplasia 2019, 24, 111–123. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients With Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- Chae, Y.K.; Arya, A.; Malecek, M.K.; Shin, D.S.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Altman, J.K.; Platanias, L.; et al. Repurposing metformin for cancer treatment: Current clinical studies. Oncotarget 2016, 7, 40767–40780. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Iwaya, C.; Kawanami, T.; Hamaguchi, Y.; Horikawa, T.; Shigeoka, T.; Yanase, T.; Kawanami, D.; Nomiyama, T. Combined treatment with glucagon-like peptide-1 receptor agonist exendin-4 and metformin attenuates breast cancer growth. Diabetol. Int. 2022, 13, 480–492. [Google Scholar] [CrossRef]
- Iwaya, C.; Nomiyama, T.; Komatsu, S.; Kawanami, T.; Tsutsumi, Y.; Hamaguchi, Y.; Horikawa, T.; Yoshinaga, Y.; Yamashita, S.; Tanaka, T.; et al. Exendin-4, a Glucagonlike Peptide-1 Receptor Agonist, Attenuates Breast Cancer Growth by Inhibiting NF-κB Activation. Endocrinology 2017, 158, 4218–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Q.; Huang, Z.; Li, Q.; Liu, D.; Wang, P.; Wang, K.; Li, J.; Cao, W.; Deng, W.; Wu, K.; et al. A Novel Metabolic Reprogramming Strategy for the Treatment of Diabetes-Associated Breast Cancer. Adv. Sci. 2022, 9, e2102303. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Chaube, B.; Mayengbam, S.S.; Singh, A.; Malvi, P.; Mohammad, N.; Deb, A.; Bhat, M.K. Metformin induced lactic acidosis impaired response of cancer cells towards paclitaxel and doxorubicin: Role of monocarboxylate transporter. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166011. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2013, 1835, 164–169. [Google Scholar] [CrossRef]
- Silva, C.; Andrade, N.; Guimarães, J.T.; Patrício, E.; Martel, F. The in vitro effect of the diabetes-associated markers insulin, leptin and oxidative stress on cellular characteristics promoting breast cancer progression is GLUT1-dependent. Eur. J. Pharmacol. 2021, 898, 173980. [Google Scholar] [CrossRef]
- Wu, Q.; Ba-Alawi, W.; Deblois, G.; Cruickshank, J.; Duan, S.; Lima-Fernandes, E.; Haight, J.; Tonekaboni, S.A.M.; Fortier, A.M.; Kuasne, H.; et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat. Commun. 2020, 11, 4205. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Martel, F. Targeting Glucose Transporters for Breast Cancer Therapy: The Effect of Natural and Synthetic Compounds. Cancers 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uldry, M.; Ibberson, M.; Hosokawa, M.; Thorens, B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002, 524, 199–203. [Google Scholar] [CrossRef]
- Wu, K.-H.; Ho, C.-T.; Chen, Z.-F.; Chen, L.-C.; Whang-Peng, J.; Lin, T.-N.; Ho, Y.-S. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J. Food Drug Anal. 2018, 26, 221–231. [Google Scholar] [CrossRef]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimaraes, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, V.; Gitenay, D.; Fritsch, S.; Bonnet, S.; Győrffy, B.; Jalaguier, S.; Linares, L.K.; Cavaillès, V.; Teyssier, C. RIP140 inhibits glycolysis-dependent proliferation of breast cancer cells by regulating GLUT3 expression through transcriptional crosstalk between hypoxia induced factor and p53. Cell. Mol. Life Sci. 2022, 79, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Yang, C.C.; Kou, T.C.; Yang, C.E.; Dai, J.Z.; Chen, C.L.; Lin, C.W. Overexpression of GLUT3 promotes metastasis of triple-negative breast cancer by modulating the inflammatory tumor microenvironment. J. Cell. Physiol. 2021, 236, 4669–4680. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.; Osorio, F.G.; Morán, J.; Cabello, E.; Alonso, A.; Freije, J.M.; González, C. Loss of GLUT4 induces metabolic reprogramming and impairs viability of breast cancer cells. J. Cell. Physiol. 2015, 230, 191–198. [Google Scholar] [CrossRef]
- Stenbit, A.E.; Tsao, T.S.; Li, J.; Burcelin, R.; Geenen, D.L.; Factor, S.M.; Houseknecht, K.; Katz, E.B.; Charron, M.J. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 1997, 3, 1096–1101. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Khalil, M.I.; Gan, S.H. Metabolic Control of Type 2 Diabetes by Targeting the GLUT4 Glucose Transporter: Intervention Approaches. Curr. Pharm. Des. 2016, 22, 3034–3049. [Google Scholar] [CrossRef]
- Rogers, S.; Macheda, M.L.; Docherty, S.E.; Carty, M.D.; Henderson, M.A.; Soeller, W.C.; Gibbs, E.M.; James, D.E.; Best, J.D. Identification of a novel glucose transporter-like protein-GLUT-12. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E733–E738. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Ran, F.; Liu, J.; Lin, J.; Hao, X.; Ding, L.; Ye, Q. Let-7a-5p inhibits triple-negative breast tumor growth and metastasis through GLUT12-mediated warburg effect. Cancer Lett. 2020, 495, 53–65. [Google Scholar] [CrossRef]
- Matsui, C.; Takatani-Nakase, T.; Maeda, S.; Nakase, I.; Takahashi, K. Potential Roles of GLUT12 for Glucose Sensing and Cellular Migration in MCF-7 Human Breast Cancer Cells Under High Glucose Conditions. Anticancer Res. 2017, 37, 6715–6722. [Google Scholar] [CrossRef]
- Purcell, S.H.; Aerni-Flessner, L.B.; Willcockson, A.R.; Diggs-Andrews, K.A.; Fisher, S.J.; Moley, K.H. Improved insulin sensitivity by GLUT12 overexpression in mice. Diabetes 2011, 60, 1478–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.B.; Fenton, R.A.; Rieg, T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Komatsu, S.; Nomiyama, T.; Numata, T.; Kawanami, T.; Hamaguchi, Y.; Iwaya, C.; Horikawa, T.; Fujimura-Tanaka, Y.; Hamanoue, N.; Motonaga, R.; et al. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr. J. 2020, 67, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhu, J.; Yu, S.J.; Ma, H.L.; Chen, J.; Ding, X.F.; Chen, G.; Liang, Y.; Zhang, Q. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharm. 2020, 132, 110821. [Google Scholar] [CrossRef]
- Nasiri, A.R.; Rodrigues, M.R.; Li, Z.; Leitner, B.P.; Perry, R.J. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Akingbesote, N.D.; Norman, A.; Zhu, W.; Halberstam, A.A.; Zhang, X.; Foldi, J.; Lustberg, M.B.; Perry, R.J. A precision medicine approach to metabolic therapy for breast cancer in mice. Commun. Biol. 2022, 5, 478. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Onishi, A.; Koepsell, H.; Vallon, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. Targets 2016, 20, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Zambrowicz, B.; Freiman, J.; Brown, P.M.; Frazier, K.S.; Turnage, A.; Bronner, J.; Ruff, D.; Shadoan, M.; Banks, P.; Mseeh, F.; et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharm. 2012, 92, 158–169. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Ferrannini, E.; Umpierrez, G.E.; Peters, A.L.; Rosenstock, J.; Carroll, A.K.; Lapuerta, P.; Banks, P.; Agarwal, R. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and severe renal impairment. Diabetes Obes. Metab. 2021, 23, 2632–2642. [Google Scholar] [CrossRef]
- Niu, X.; Ma, J.; Li, J.; Gu, Y.; Yin, L.; Wang, Y.; Zhou, X.; Wang, J.; Ji, H.; Zhang, Q. Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis. 2021, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ertay, A.; Peng, P.; Li, J.; Liu, D.; Xiong, H.; Zou, Y.; Qiu, H.; Hancock, D.; Yuan, X.; et al. SGLT1 is required for the survival of triple-negative breast cancer cells via potentiation of EGFR activity. Mol. Oncol. 2019, 13, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, H.; Niu, X.; Yin, L.; Wang, Y.; Gu, Y.; Li, D.; Zhang, H.; Lu, M.; Zhang, F.; et al. Sodium-Dependent Glucose Transporter 1 (SGLT1) Stabled by HER2 Promotes Breast Cancer Cell Proliferation by Activation of the PI3K/Akt/mTOR Signaling Pathway in HER2+ Breast Cancer. Dis. Mrk. 2020, 2020, 6103542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family--role and regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Baenke, F.; Dubuis, S.; Brault, C.; Weigelt, B.; Dankworth, B.; Griffiths, B.; Jiang, M.; Mackay, A.; Saunders, B.; Spencer-Dene, B.; et al. Functional screening identifies MCT4 as a key regulator of breast cancer cell metabolism and survival. J. Pathol. 2015, 237, 152–165. [Google Scholar] [CrossRef]
- Pinheiro, C.; Albergaria, A.; Paredes, J.; Sousa, B.; Dufloth, R.; Vieira, D.; Schmitt, F.; Baltazar, F. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology 2010, 56, 860–867. [Google Scholar] [CrossRef]
- Benyahia, Z.; Blackman, M.; Hamelin, L.; Zampieri, L.X.; Capeloa, T.; Bedin, M.L.; Vazeille, T.; Schakman, O.; Sonveaux, P. In Vitro and In Vivo Characterization of MCT1 Inhibitor AZD3965 Confirms Preclinical Safety Compatible with Breast Cancer Treatment. Cancers 2021, 13, 569. [Google Scholar] [CrossRef]
- Silva, A.; Antunes, B.; Batista, A.; Pinto-Ribeiro, F.; Baltazar, F.; Afonso, J. In Vivo Anticancer Activity of AZD3965: A Systematic Review. Molecules 2021, 27, 181. [Google Scholar] [CrossRef]
- Padilla, J.; Lee, B.S.; Zhai, K.; Rentz, B.; Bobo, T.; Dowling, N.M.; Lee, J. A Heme-Binding Transcription Factor BACH1 Regulates Lactate Catabolism Suggesting a Combined Therapy for Triple-Negative Breast Cancer. Cells 2022, 11, 1177. [Google Scholar] [CrossRef]
- Duan, X.; Xie, Y.; Yu, J.; Hu, X.; Liu, Z.; Li, N.; Qin, J.; Lan, L.; Yuan, M.; Pan, Z.; et al. MCT4/Lactate Promotes PD-L1 Glycosylation in Triple-Negative Breast Cancer Cells. J. Oncol. 2022, 2022, 3659714. [Google Scholar] [CrossRef]
- Luo, E.; Wang, D.; Yan, G.; Qiao, Y.; Zhu, B.; Liu, B.; Hou, J.; Tang, C. The NF-κB/miR-425-5p/MCT4 axis: A novel insight into diabetes-induced endothelial dysfunction. Mol. Cell Endocrinol. 2020, 500, 110641. [Google Scholar] [CrossRef] [PubMed]
- Nadai, T.; Narumi, K.; Furugen, A.; Saito, Y.; Iseki, K.; Kobayashi, M. Pharmacological Inhibition of MCT4 Reduces 4-Hydroxytamoxifen Sensitivity by Increasing HIF-1α Protein Expression in ER-Positive MCF-7 Breast Cancer Cells. Biol. Pharm. Bull. 2021, 44, 1247–1253. [Google Scholar] [CrossRef]
- Ekyalongo, R.C.; Yee, D. Revisiting the IGF-1R as a breast cancer target. NPJ Precis. Oncol. 2017, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H. Prolonged use of human insulin increases breast cancer risk in Taiwanese women with type 2 diabetes. BMC Cancer 2015, 15, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platts, J. Insulin therapy and cancer risk in diabetes mellitus. Clin. Med. 2010, 10, 509–512. [Google Scholar] [CrossRef]
- Sarkissyan, S.; Sarkissyan, M.; Wu, Y.; Cardenas, J.; Koeffler, H.P.; Vadgama, J.V. IGF-1 Regulates Cyr61 Induced Breast Cancer Cell Proliferation and Invasion. PLoS ONE 2014, 9, e103534. [Google Scholar] [CrossRef] [Green Version]
- Hellinger, J.W.; Hüchel, S.; Goetz, L.; Bauerschmitz, G.; Emons, G.; Gründker, C. Inhibition of CYR61-S100A4 Axis Limits Breast Cancer Invasion. Front. Oncol. 2019, 9, 1074. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Huo, R.; Wang, L.; Zhou, Z.; Sun, Y.; Shen, B.; Wang, R.; Li, N. A novel anti-Cyr61 antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2012, 61, 677–687. [Google Scholar] [CrossRef]
- Cao, J.; Yee, D. Disrupting Insulin and IGF Receptor Function in Cancer. Int. J. Mol. Sci. 2021, 22, 555. [Google Scholar] [CrossRef]
- Wenbin, K.; Xiaoqin, L.; Qiuchan, D.; Xinwen, Z.; Xiaoqin, X.; Fangyuan, S.; Dabao, H.; Shuangjiu, Z. Development of a novel insulin receptor (IR) antagonist that exhibits anti-breast tumor activity. Hum. Cell 2020, 33, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, R.; Bitton-Worms, K.; Caspi, A.; Shen-Orr, Z.; LeRoith, D. Investigating new therapeutic strategies targeting hyperinsulinemia’s mitogenic effects in a female mouse breast cancer model. Endocrinology 2013, 154, 1701–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, N.; Márquez-Garbán, D.; Rogers, B.; Austin, D.; Foos, K.; Tong, A.; Adams, D.; Vadgama, J.; Brecht, M.-L.; Pietras, R. Dual Therapy with Insulin-Like Growth Factor-I Receptor/Insulin Receptor (IGF1R/IR) and Androgen Receptor (AR) Antagonists Inhibits Triple-Negative Breast Cancer Cell Migration In Vitro. SPG BioMed 2019. [Google Scholar] [CrossRef]
- Hamilton, N.M.; Marquez-Garban, D.C.; Burton, L.P.; Comin-Anduix, B.; Garcia, A.J.; Vadgama, J.V. Abstract LB-391: Combination of insulin-like growth factor-1 receptor/insulin receptor (IGF1R/IR) antagonist with anti-PD-L1 antibody blocks triple-negative breast cancer (TNBC) progression. Cancer Res. 2020, 80, LB-391. [Google Scholar] [CrossRef]
- Aguirre, G.A.; De Ita, J.R.; de la Garza, R.G.; Castilla-Cortazar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J. Transl. Med. 2016, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Zhang, C.; Le, A. Diabetes and cancer: The epidemiological and metabolic associations. In The Heterogeneity of Cancer Metabolism; Le, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 217–227. [Google Scholar]
- Melnik, B.C. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J. Diabetes 2012, 3, 38–53. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar]
- Sato, M.; Harada-Shoji, N.; Toyohara, T.; Soga, T.; Itoh, M.; Miyashita, M.; Tada, H.; Amari, M.; Anzai, N.; Furumoto, S.; et al. L-type amino acid transporter 1 is associated with chemoresistance in breast cancer via the promotion of amino acid metabolism. Sci. Rep. 2021, 11, 589. [Google Scholar] [CrossRef]
- Shindo, H.; Harada-Shoji, N.; Ebata, A.; Sato, M.; Soga, T.; Miyashita, M.; Tada, H.; Kawai, M.; Kosaka, S.; Onuki, K.; et al. Targeting Amino Acid Metabolic Reprogramming via L-Type Amino Acid Transporter 1 (LAT1) for Endocrine-Resistant Breast Cancer. Cancers 2021, 13, 4375. [Google Scholar] [CrossRef]
- Bo, T.; Kobayashi, S.; Inanami, O.; Fujii, J.; Nakajima, O.; Ito, T.; Yasui, H. LAT1 inhibitor JPH203 sensitizes cancer cells to radiation by enhancing radiation-induced cellular senescence. Transl. Oncol. 2021, 14, 101212. [Google Scholar] [CrossRef]
- van Geldermalsen, M.; Quek, L.-E.; Turner, N.; Freidman, N.; Pang, A.; Guan, Y.F.; Krycer, J.R.; Ryan, R.; Wang, Q.; Holst, J. Benzylserine inhibits breast cancer cell growth by disrupting intracellular amino acid homeostasis and triggering amino acid response pathways. BMC Cancer 2018, 18, 689. [Google Scholar] [CrossRef] [Green Version]
- Krokowski, D.; Han, J.; Saikia, M.; Majumder, M.; Yuan, C.L.; Guan, B.J.; Bevilacqua, E.; Bussolati, O.; Bröer, S.; Arvan, P.; et al. A self-defeating anabolic program leads to β-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J. Biol. Chem. 2013, 288, 17202–17213. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Beltran, V.D.; Chan, S.M.; Brown, J.R.; Bevington, A.; Herbert, T.P. System-L amino acid transporters play a key role in pancreatic β-cell signalling and function. J. Mol. Endocrinol. 2016, 56, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, Y.; Li, D.; Fu, L.; Zhang, X.; Bao, Y.; Zheng, L. Review of the Correlation of LAT1 With Diseases: Mechanism and Treatment. Front Chem. 2020, 8, 564809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.-H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Chen, S.; Li, Y.; Han, H.; Gao, J.; Liu, G.; Wu, X.; Li, T.; Woo Kim, S.; et al. Metabolic Regulation of Methionine Restriction in Diabetes. Mol. Nutr. Food Res. 2018, 62, e1700951. [Google Scholar] [CrossRef]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malin, D.; Lee, Y.; Chepikova, O.; Strekalova, E.; Carlson, A.; Cryns, V.L. Methionine restriction exposes a targetable redox vulnerability of triple-negative breast cancer cells by inducing thioredoxin reductase. Breast Cancer Res. Treat. 2021, 190, 373–387. [Google Scholar] [CrossRef]
- Lim, H.I.; Sun, Y.U.; Han, Q.; Yamamoto, J.; Hoffman, R.M. Efficacy of Oral Recombinant Methioninase and Eribulin on a PDOX Model of Triple-negative Breast Cancer (TNBC) Liver Metastasis. In Vivo 2021, 35, 2531–2534. [Google Scholar] [CrossRef]
- Lim, H.I.; Hamada, K.; Yamamoto, J.; Han, Q.; Tan, Y.; Choi, H.J.; Nam, S.J.; Bouvet, M.; Hoffman, R.M. Oral Methioninase Inhibits Recurrence in a PDOX Mouse Model of Aggressive Triple-negative Breast Cancer. In Vivo 2020, 34, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Han, Q.; Tan, Y.; Sugisawa, N.; Yamamoto, J.; Nishino, H.; Inubushi, S.; Sun, Y.U.; Zhu, G.; Lim, H.; et al. Oral Recombinant Methioninase Inhibits Diabetes Onset in Mice on a High-fat Diet. In Vivo 2020, 34, 973–978. [Google Scholar] [CrossRef]
- Tang, X.; Ding, C.K.; Wu, J.; Sjol, J.; Wardell, S.; Spasojevic, I.; George, D.; McDonnell, D.P.; Hsu, D.S.; Chang, J.T.; et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene 2017, 36, 4235–4242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alothaim, T.; Charbonneau, M.; Tang, X. HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast cancer cells to cysteine deprivation. Sci. Rep. 2021, 11, 10956. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cao, Y.; Wang, Y.; Li, W.; Liu, X.; Lv, Y.; Li, X.; Mi, J. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics 2017, 7, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.K.; Velusamy, T.; Croad, J.L.; Rains, J.L.; Bull, R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits NF-kappaB activation in the livers of Zucker diabetic rats. Free Radic. Biol. Med. 2009, 46, 1633–1638. [Google Scholar] [CrossRef] [Green Version]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Tappia, P.S.; Xu, Y.J.; Rodriguez-Leyva, D.; Aroutiounova, N.; Dhalla, N.S. Cardioprotective effects of cysteine alone or in combination with taurine in diabetes. Physiol. Res. 2013, 62, 171–178. [Google Scholar] [CrossRef]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D., II. Glutamine and glutamate. Biomed. Pharm. 2002, 56, 446–457. [Google Scholar] [CrossRef]
- Miller, R.A.; Shi, Y.; Lu, W.; Pirman, D.A.; Jatkar, A.; Blatnik, M.; Wu, H.; Cárdenas, C.; Wan, M.; Foskett, J.K.; et al. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat. Med. 2018, 24, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Qorbani, M.; Heshmat, R.; Larijani, B.; Hosseini, S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015, 31, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Mansour, A.; Asayesh, H.; Djalalinia, S.; Mahdavi Gorabi, A.; Ochi, F.; Qorbani, M. Effect of glutamine supplementation on cardiometabolic risk factors and inflammatory markers: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2021, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Hosios, A.M.; Hecht, V.C.; Danai, L.V.; Johnson, M.O.; Rathmell, J.C.; Steinhauser, M.L.; Manalis, S.R.; Vander Heiden, M.G. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev. Cell 2016, 36, 540–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.-L.; Durán, R.V. Glutamine metabolism in cancer therapy. Cancer Drug Resist. 2018, 1, 126–138. [Google Scholar] [CrossRef] [Green Version]
- van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.J.; Khelifa, S.; Ratnikov, B.; Scott, D.A.; Feng, Y.; Parisi, F.; Ruller, C.; Lau, E.; Kim, H.; Brill, L.M.; et al. Regulation of Glutamine Carrier Proteins by RNF5 Determines Breast Cancer Response to ER Stress-Inducing Chemotherapies. Cancer Cell 2015, 27, 354–369. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.M., Jr. Arginine: Beyond protein. Am. J. Clin. Nutr. 2006, 83, 508S–512S. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or NOt? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef] [PubMed]
- Too, C.K.L.; Abdelmagid, S.A. l-Arginine Uptake and Its Role in the Survival of Breast Cancer Cells. In L-Arginine in Clinical Nutrition; Patel, V.B., Preedy, V.R., Rajendram, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 253–268. [Google Scholar]
- Yao, S.; Janku, F.; Koenig, K.; Tsimberidou, A.M.; Piha-Paul, S.A.; Shi, N.; Stewart, J.; Johnston, A.; Bomalaski, J.; Meric-Bernstam, F.; et al. Phase 1 trial of ADI-PEG 20 and liposomal doxorubicin in patients with metastatic solid tumors. Cancer Med. 2022, 11, 340–347. [Google Scholar] [CrossRef]
- Nasreddine, G.; El-Sibai, M.; Abi-Habib, R.J. Cytotoxicity of [HuArgI (co)-PEG5000]-induced arginine deprivation to ovarian Cancer cells is autophagy dependent. Investig. New Drugs 2020, 38, 10–19. [Google Scholar] [CrossRef] [PubMed]
- El-Mais, N.; Fakhoury, I.; Al Haddad, M.; Nohra, S.; Abi-Habib, R.; El-Sibai, M. Human Recombinant Arginase I [HuArgI(Co)-PEG5000]-Induced Arginine Depletion Inhibits Pancreatic Cancer Cell Migration and Invasion Through Autophagy. Pancreas 2021, 50, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.L.; Kim, R.; Galante, J.; Parsons, C.M.; Virudachalam, S.; Kung, H.-J.; Bold, R.J. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int. J. Cancer 2008, 123, 1950–1955. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Feng, Y.; Zhang, Y.; Zhu, X.; Jin, F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer 2016, 16, 343. [Google Scholar] [CrossRef] [Green Version]
- Jahani, M.; Azadbakht, M.; Norooznezhad, F.; Mansouri, K. L-arginine alters the effect of 5-fluorouracil on breast cancer cells in favor of apoptosis. Biomed. Pharmacother. 2017, 88, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Loh, E.; Roddy, M.A.; Currie, K.E.; Creager, M.A. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation 1994, 89, 2035–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jude, E.B.; Dang, C.; Boulton, A.J. Effect of L-arginine on the microcirculation in the neuropathic diabetic foot in Type 2 diabetes mellitus: A double-blind, placebo-controlled study. Diabet. Med. 2010, 27, 113–116. [Google Scholar] [CrossRef]
- Claybaugh, T.; Decker, S.; McCall, K.; Slyvka, Y.; Steimle, J.; Wood, A.; Schaefer, M.; Thuma, J.; Inman, S. L-Arginine Supplementation in Type II Diabetic Rats Preserves Renal Function and Improves Insulin Sensitivity by Altering the Nitric Oxide Pathway. Int. J. Endocrinol. 2014, 2014, 171546. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.-K.; Jang, H.G.; Jha, A.K.; et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Mullarky, E.; Lucki, N.C.; Beheshti Zavareh, R.; Anglin, J.L.; Gomes, A.P.; Nicolay, B.N.; Wong, J.C.Y.; Christen, S.; Takahashi, H.; Singh, P.K.; et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. USA 2016, 113, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Yuan, L.; Shi, X.W.; Feng, K.R.; Lan, X.; Huang, C.; Lin, G.Q.; Tian, P.; Huang, M.; Tang, S.; et al. Discovery of PHGDH inhibitors by virtual screening and preliminary structure-activity relationship study. Bioorg. Chem. 2022, 121, 105705. [Google Scholar] [CrossRef]
- Pacold, M.E.; Brimacombe, K.R.; Chan, S.H.; Rohde, J.M.; Lewis, C.A.; Swier, L.J.Y.M.; Possemato, R.; Chen, W.W.; Sullivan, L.B.; Fiske, B.P.; et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 2016, 12, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Spillier, Q.; Frédérick, R. Phosphoglycerate dehydrogenase (PHGDH) inhibitors: A comprehensive review 2015–2020. Expert Opin. Ther. Pat. 2021, 31, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Drábková, P.; Šanderová, J.; Kovařík, J.; Kanďár, R. An Assay of Selected Serum Amino Acids in Patients with Type 2 Diabetes Mellitus. Adv. Clin. Exp. Med. 2015, 24, 447–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, L.J.; Buschard, K. L-serine: A neglected amino acid with a potential therapeutic role in diabetes. Apmis 2019, 127, 655–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, K.; Usui, I.; Yaku, K.; Hirabayashi, Y.; Tobe, K.; Nakagawa, T. Deletion of PHGDH in adipocytes improves glucose intolerance in diet-induced obese mice. Biochem. Biophys. Res. Commun. 2018, 504, 309–314. [Google Scholar] [CrossRef]
- Suwandhi, L.; Hausmann, S.; Braun, A.; Gruber, T.; Heinzmann, S.S.; Gálvez, E.J.C.; Buck, A.; Legutko, B.; Israel, A.; Feuchtinger, A.; et al. Chronic d-serine supplementation impairs insulin secretion. Mol. Metab. 2018, 16, 191–202. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6267, Asparagine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Asparagine (accessed on 25 November 2022).
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef] [Green Version]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.-O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018, 554, 378–381. [Google Scholar] [CrossRef]
- Yang, H.; He, X.; Zheng, Y.; Feng, W.; Xia, X.; Yu, X.; Lin, Z. Down-regulation of asparagine synthetase induces cell cycle arrest and inhibits cell proliferation of breast cancer. Chem. Biol. Drug Des. 2014, 84, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Radadiya, A.; Bisson, C.; Wenzel, S.; Nordin, B.E.; Martínez-Márquez, F.; Imasaki, T.; Sedelnikova, S.E.; Coricello, A.; Baumann, P.; et al. High-resolution crystal structure of human asparagine synthetase enables analysis of inhibitor binding and selectivity. Commun. Biol. 2019, 2, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, G.S.; Desai, K.; Darwech, M.; Naim, U.; Jin, X.; Adak, S.; Harroun, N.; Sanchez, L.A.; Semenkovich, C.F.; Zayed, M.A. Circulating serum fatty acid synthase is elevated in patients with diabetes and carotid artery stenosis and is LDL-associated. Atherosclerosis 2019, 287, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Singh, S.B.; Wang, J.; Chung, C.C.; Salituro, G.; Karanam, B.V.; Lee, S.H.; Powles, M.; Ellsworth, K.P.; Lassman, M.E.; et al. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 5378–5383. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Yuan, L.X.; Boulbes, D.; Baek, J.M.; Wang, Y.N.; Gomez-Cabello, D.; Hawke, D.H.; Yeung, S.C.; Lee, M.H.; Hortobagyi, G.N.; et al. Fatty acid synthase phosphorylation: A novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010, 12, R96. [Google Scholar] [CrossRef] [Green Version]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Menendez, J.A.; Mehmi, I.; Papadimitropoulou, A.; Vander Steen, T.; Cuyàs, E.; Verdura, S.; Espinoza, I.; Vellon, L.; Atlas, E.; Lupu, R. Fatty Acid Synthase Is a Key Enabler for Endocrine Resistance in Heregulin-Overexpressing Luminal B-Like Breast Cancer. Int. J. Mol. Sci. 2020, 21, 7661. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin. Ther. Targets 2017, 21, 1001–1016. [Google Scholar] [CrossRef]
- Schroeder, B.; Vander Steen, T.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Regan, K.; Cuyàs, E.; Meng, X.W.; Verdura, S.; et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, A.A.; Al-Barzinji, R. Soluble CD36 Concentration in Diabetic Hypertensive Patients with Coronary Atherosclerosis. Cell Mol. Biol. 2022, 68, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Guo, R.K.; Li, Z.C.; Zhang, R.; Li, L.; Li, H.Y.; Zhang, J.L.; Han, Q.Q.; Liu, F. [Association of Serum Soluble CD36 with Clinical Variables in Diabetic Patients with Chronic Kidney Diseas]. Sichuan Da Xue Xue Bao Yi Xue Ban 2018, 49, 414–419. [Google Scholar]
- Puchałowicz, K.; Rać, M.E. The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring. Cells 2020, 9, 1877. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, F.; Di Cosimo, S.; Verderio, P.; Ciniselli, C.M.; Pizzamiglio, S.; Castagnoli, L.; Dugo, M.; Galbardi, B.; Salgado, R.; Loi, S.; et al. Predictive role of CD36 expression in HER2-positive breast cancer patients receiving neoadjuvant trastuzumab. J. Natl. Cancer Inst. 2022, 144, 1720–1727. [Google Scholar] [CrossRef]
- Feng, W.W.; Wilkins, O.; Bang, S.; Ung, M.; Li, J.; An, J.; Del Genio, C.; Canfield, K.; Di Renzo, J.; Wells, W.; et al. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 2019, 29, 3405–3420.e3405. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhi, Z.; Wang, C.; Xing, H.; Song, G.; Yu, X.; Zhu, Y.; Wang, X.; Zhang, X.; Di, Y. Exogenous lipids promote the growth of breast cancer cells via CD36. Oncol. Rep. 2017, 38, 2105–2115. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.; Rychahou, P.G.; He, D.; Jafari, N.; Wang, C.; Lee, E.Y.; Weiss, H.L.; Evers, B.M.; Zaytseva, Y.Y. Inhibition of Fatty Acid Synthase Upregulates Expression of CD36 to Sustain Proliferation of Colorectal Cancer Cells. Front. Oncol. 2020, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Luby, A.; Alves-Guerra, M.C. Targeting Metabolism to Control Immune Responses in Cancer and Improve Checkpoint Blockade Immunotherapy. Cancers 2021, 13, 5912. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, O.S.; Pethusamy, K.; Srivastava, T.P.; Talukdar, J.; Alqahtani, M.S.; Abbas, M.; Dhar, R.; Karmakar, S. The metabolic addiction of cancer stem cells. Front. Oncol. 2022, 12, 955892. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Y.; Atefi, M.; Liu, Y.; Elshimali, Y.; Vadgama, J.V. Lactate, a Neglected Factor for Diabetes and Cancer Interaction. Mediators Inflamm. 2016, 2016, 6456018. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.; Wallis, R.; Soilleux, E.J.; Townsend, P.; Zehnder, D.; Tan, B.K.; Sim, R.B.; Lehnert, H.; Randeva, H.S.; Mitchell, D.A. High glucose disrupts oligosaccharide recognition function via competitive inhibition: A potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology 2011, 216, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Shcheglova, T.; Makker, S.; Tramontano, A. Reactive immunization suppresses advanced glycation and mitigates diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Vrdoljak, A.; Trescec, A.; Benko, B.; Hecimovic, D.; Simic, M. In vitro glycation of human immunoglobulin G. Clin. Chim. Acta 2004, 345, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Tonani, R.; Fedele, D.; Garbeglio, M.; Senesi, A.; Seraglia, R.; Favretto, D.; Traldi, P. Non-enzymatic glycation of IgG: An in vivo study. Horm. Metab. Res. 2002, 34, 260–264. [Google Scholar] [CrossRef]
- Kumar, N.P.; Sridhar, R.; Nair, D.; Banurekha, V.V.; Nutman, T.B.; Babu, S. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology 2015, 144, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Moura, J.; Rodrigues, J.; Goncalves, M.; Amaral, C.; Lima, M.; Carvalho, E. Impaired T-cell differentiation in diabetic foot ulceration. Cell Mol. Immunol. 2017, 14, 758–769. [Google Scholar] [CrossRef] [Green Version]
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]


| Title | Status | Study Results | Conditions | Interventions | Phases | NCT Number | |
|---|---|---|---|---|---|---|---|
| 1 | Alpelisib, Fulvestrant and Dapagliflozin for the Treatment of HR+, HER2-, PIK3CA Mutant Metastatic Breast Cancer | Recruiting | No Results Available | Metastatic BC HER2-negative BC | Dapagliflozin 10 Mg Tab | Phase 2 | NCT05025735 |
| 2 | A Phase 1b/2 Study of Serabelisib in Combination with Canagliflozin in Patients with Advanced Solid Tumors | Unknown status | No Results Available | BC Endometrial Cancer Lung Cancer Colorectal Cancer Head and Neck Cancer | Serabelisib Canagliflozin 300 mg | Phase 1 Phase 2 | NCT04073680 |
| 3 | Preventing High Blood Sugar in People Being Treated for Metastatic Breast Cancer | Recruiting | No Results Available | BC BC Stage IV Metastatic BC | Dietary Supplement: Ketogenic Diet Dietary Supplement: Low Carbohydrate Diet Drug: Alpelisib Drug: Fulvestrant Drug: Canagliflozin | Phase 2 | NCT05090358 |
| 4 | Study of Safety and Efficacy of Dapagliflozin + Metformin XR Versus Metformin XR in Participants With HR+, HER2-, Advanced Breast Cancer While on Treatment with Alpelisib and Fulvestrant | Recruiting | No Results Available | BC | Alpelisib Fulvestrant Metformin XR Dapagliflozin + metformin XR Dapagliflozin | Phase 2 | NCT04899349 |
| Title | Status | Study Results | Conditions | Interventions | Phases | NCT Number | |
|---|---|---|---|---|---|---|---|
| 1 | Arginine Metabolism in Pediatric Type 2 Diabetes | Recruiting | No Results Available | Type 2 Diabetes | Other: Stable isotope infusion, oral glucose ingestion, intravenous arginine bolus | Not Applicable | NCT05477134 |
| 2 | SOAR-2: Intervening in Obesity Through Reduction of Dietary Branched Chain Amino Acids | Withdrawn | No Results Available | Obesity Diabetes | Control diet Dietary Supplement: Low branched-chain amino acids (BCAA) diet Dietary Supplement: Low protein diet | Early Phase 1 | NCT04424537 |
| 3 | Targeting Glutamine Metabolism to Prevent Diabetic Cardiovascular Complications | Recruiting | No Results Available | Glutamine Diabetic Cardiovascular Complications | Biological: Bio collection | NCT04353869 | |
| 4 | Effect of L-arginine on Microcirculation, Myogenesis and Angiogenesis Associated With Aging, Sarcopenia and Diabetes | Completed | No Results Available | Aging Sarcopenia Type 2 Diabetes Microcirculation | L-arginine Maltodextrin | Not Applicable | NCT04112875 |
| 5 | The Effect of Pharmaceutical Grade L-glutamine (Endari) on Glycemic Control in Patients With Diabetes Mellitus Type II | Unknown status | No Results Available | Diabetes Mellitus, Type 2 | Drug: L-glutamine Other: No L-glutamine | Phase 1 | NCT03947879 |
| 6 | Study of CB-839 (Telaglenastat) in Combination With Talazoparib in Patients With Solid Tumors | Terminated | Has Results | Solid Tumor Clear Cell Renal Cell Carcinoma TNBC Colorectal Cancer CRC|RCC| ccRCC | Drug: CB-839 Drug: Talazoparib | Phase 1 Phase 2 | NCT03875313 |
| 7 | Sulfasalazine in Decreasing Opioids Requirements in Breast Cancer Patients | Recruiting | No Results Available | Breast Cancer Chronic Pain Due to Malignancy (Finding) | Drug: Sulfasalazine Drug: Placebos | Phase 2 | NCT03847311 |
| 8 | ONC201 With a Methionine-Restricted Diet in Patients with Metastatic Triple Negative Breast Cancer | Terminated | Has Results | TNBC | Drug: Akt/ERK Inhibitor ONC201 Dietary Supplement: Methionine-Restricted Diet | Phase 2 | NCT03733119 |
| 9 | Study of Eryaspase in Combination with Chemotherapy Versus Chemotherapy Alone for the Treatment of TNBC (TRYbeCA-2) | Terminated | No Results Available | TNBC | Eryaspase (L-asparaginase encapsulated in red blood cells) Gemcitabine Carboplatin | Phase 2 Phase 3 | NCT03674242 |
| 10 | Methionine-Restricted Diet to Potentiate the Effects of Radiation Therapy | Suspended | No Results Available | Cancer Lung Cancer Prostate Cancer BC | Dietary Supplement: Methionine-restricted diet | Not Applicable | NCT03574194 |
| 11 | Study of Arginine and Nitric Oxide in Patients with Diabetes | Completed | No Results Available | Ketosis Prone Diabetes | Dietary Supplement: Citrulline Dietary Supplement: Alanine | Not Applicable | NCT03566524 |
| 12 | Effect of BKR-013 on Average Daily Glucose Levels | Completed | No Results Available | Type 2 DM | Other: BKR-013 or Placebo | Not Applicable | NCT03382015 |
| 13 | Exercise Snacks and Glutamine to Improve Glucose Control in Adolescents with Type 1 Diabetes | Unknown status | No Results Available | DM, Type 1 Autoimmune Diseases DM Endocrine System Diseases Glucose Metabolism Disorders Immune System Diseases Metabolic Diseases | Drug: Glutamine vs. Placebo Other: Exercise | Not Applicable | NCT03199638 |
| 14 | A Window of Opportunity Study of Methionine Deprivation in Triple Negative Breast Cancer | Terminated | No Results Available | BC TNBC | Dietary Supplement: hominex-2 | Phase 2 | NCT03186937 |
| 15 | Study of CB-839 in Combination w/Paclitaxel in Participants of African Ancestry and Non-African Ancestry with Advanced Triple Negative Breast Cancer (TNBC) | Completed | Has Results | TNBC | Drug: Paclitaxel Drug: CB-839 | Phase 2 | NCT03057600 |
| 16 | Arginase Inhibition and Microvascular Endothelial Function in Type 2 Diabetes | Completed | No Results Available | Type 2 DM | Other: N-hydroxy-nor-L-arginine | Phase 1 Phase 2 | NCT02687152 |
| 17 | Treatment of Type 2 Diabetes with Immunonutrients | Completed | No Results Available | DM, Type 2 | Dietary Supplement: Arginine and fish oil | Not Applicable | NCT02462863 |
| 18 | An Extension Protocol to Evaluate Dose Comparisons of Leucine-Metformin Combinations in Type 2 Diabetic Patients | Completed | Has Results | Type 2 DM | Low Metformin Mid Metformin High Metformin Metformin | Phase 2 | NCT02435277 |
| 19 | Novel Type 2 Diabetes Mellitus Preventive Therapies | Completed | No Results Available | Diabetes | Drug: Glutamine (Pharmacological doses) Behavioral: Lifestyle change | Phase 1 | NCT02351323 |
| 20 | Dose Comparisons of Leucine-Metformin Combinations on Blood Glucose Levels in Type 2 Diabetic Patients | Completed | Has Results | Type 2 DM | Low Metformin Metformin Mid Metformin High Metformin | Phase 2 | NCT02151461 |
| 21 | Study of the Glutaminase Inhibitor CB-839 in Solid Tumors | Completed | No Results Available | Solid Tumors TNBC Non-Small Cell Lung Cancer Renal Cell Carcinoma Mesothelioma Fumarate Hydratase (FH)-Deficient Tumors Succinate Dehydrogenase (SDH)-Deficient Gastrointestinal Stromal Tumors (GIST) Succinate Dehydrogenase (SDH)-Deficient Non-gastrointestinal Stromal Tumors Tumors Harboring Isocitrate Dehydrogenase-1 (IDH1) and IDH2 Mutations Tumors Harboring Amplifications in the cMyc Gene | Drug: CB-839 Drug: Pac-CB Drug: CBE Drug: CB-Erl Drug: CBD Drug: CB-Cabo | Phase 1 | NCT02071862 |
| 22 | Arginase Inhibition in Ischemia-reperfusion Injury | Completed | No Results Available | Coronary Artery Disease Type 2 DM | Drug: N-hydroxy-nor-arginine Drug: NaCl | Phase 1 | NCT02009527 |
| 23 | Ph 1 ADI-PEG 20 Plus Doxorubicin; Patients with HER2 Negative Metastatic Breast Cancer | Completed | No Results Available | HER2 Negative Metastatic BC | Drug: ADI-PEG 20 | Phase 1 | NCT01948843 |
| 24 | L-Arginine, Vascular Response and Mechanisms | Completed | No Results Available | Hypertension DM | Dietary Supplement: L-Arginine Placebo Supplement | Phase 2 | NCT01482247 |
| 25 | Glutamine and Insulin Sensitivity in Type I Diabetes | Completed | Has Results | Type I DM | Dietary Supplement: Glutamine Placebo | Not Applicable | NCT01467063 |
| 26 | Prevention of Type 2 Diabetes Mellitus by L-Arginine in Patients with Metabolic Syndrome | Completed | No Results Available | Metabolic Syndrome Impaired Glucose Tolerance |Insulin Resistance Endothelial Dysfunction | Drug: L-arginine Drug: Placebo | Phase 3 | NCT00917449 |
| 27 | Riluzole in Women with Stage I, Stage II, or Stage IIIA Breast Cancer | Withdrawn | No Results Available | BC | Drug: riluzole Genetic: polymorphism analysis Procedure: Axillary lymph node biopsy Digital image analysis Needle biopsy Sentinel lymph node biopsy Therapeutic conventional surgery | Phase 1 | NCT00903214 |
| 28 | Effect of Arginine on Microcirculation in Patients with Diabetes | Completed | No Results Available | Type 2 DM | Dietary Supplement: L-arginine Placebo Lactose powder | Phase 4 | NCT00902616 |
| 29 | Effects of Glutamine on GLP-1 and Insulin Secretion in Man | Completed | Has Results | Type 2 DM | Drug: Sitagliptin Drug: Placebo | Not Applicable | NCT00673894 |
| 30 | N-Acetylcysteine and Arginine Administration in Diabetic Patients | Terminated | No Results Available | Type 2 DM Hypertension | Drug: Arginine Drug: Acetylcysteine Drug: Placebo | Phase 4 | NCT00569465 |
| Title | Status | Study Results | Conditions | Interventions | Phases | URL | |
|---|---|---|---|---|---|---|---|
| 1 | Nasturtium (Tropaeolum Majus L) Intake and Biochemical Parameters in Pre-diabetic Subjects in Bogota Colombia | Completed | No Results Available | Pre Diabetes | Dietary Supplement: Nasturtium (Tropaeolum majus) | Not Applicable | NCT05346978 |
| 2 | FASN Inhibitor TVB-2640 and Trastuzumab in Combination with Paclitaxel or Endocrine Therapy for the Treatment of HER2 Positive Metastatic Breast Cancer | Recruiting | No Results Available | Advanced Breast Carcinoma HER2 Positive Breast Carcinoma Stage III Breast Cancer AJCC v7 Stage IIIA Breast Cancer AJCC v7 Stage IIIB Breast Cancer AJCC v7 Stage IIIC Breast Cancer AJCC v7 Stage IV Breast Cancer AJCC v6 and v7 | Anastrozole Exemestane FASN Inhibitor TVB-2640 Fulvestrant Letrozole Paclitaxel Trastuzumab | Phase 2 | NCT03179904 |
| 3 | CD36 in Nutrient Delivery and Its Dysfunction | Completed | No Results Available | Insulin Resistance Endothelial Dysfunction | Sildenafil Citrate | Phase 1 Phase 2 | NCT03012386 |
| 4 | Evaluation of 3-V Bioscience-2640 to Reduce de Novo Lipogenesis in Subjects with Characteristics of Metabolic Syndrome | Completed | No Results Available | Metabolic Syndrome | 3-V Bioscience-2640 | Phase 1 Phase 2 | NCT02948569 |
| 5 | Inhibiting Fatty Acid Synthase to Improve Efficacy of Neoadjuvant Chemotherapy | Completed | Has Results | BC | Omeprazole | Phase 2 | NCT02595372 |
| 6 | Polymorphisms in CD36 and STAT3 Genes and Different Dietary Interventions Among Patients with Coronary Artery Disease | Unknown status | No Results Available | Coronary Artery Disease | Dietary Supplement: Olive oil Nuts Control diet | Not Applicable | NCT02202265 |
| 7 | Metabolic and Cardiovascular Impact of CD36 Deficiency in African Americans | Completed | No Results Available | Obesity | NCT02126735 | ||
| 8 | Proof of Principle Trial to Determine if Nutritional Supplement Conjugated Linoleic Acid (CLA) Can Modulate the Lipogenic Pathway in Breast Cancer Tissue | Completed | Has Results | BC | Conjugated Linoleic Acid (CLA) | Early Phase 1 | NCT00908791 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shum, H.C.E.; Wu, K.; Vadgama, J.; Wu, Y. Potential Therapies Targeting the Metabolic Reprogramming of Diabetes-Associated Breast Cancer. J. Pers. Med. 2023, 13, 157. https://doi.org/10.3390/jpm13010157
Shum HCE, Wu K, Vadgama J, Wu Y. Potential Therapies Targeting the Metabolic Reprogramming of Diabetes-Associated Breast Cancer. Journal of Personalized Medicine. 2023; 13(1):157. https://doi.org/10.3390/jpm13010157
Chicago/Turabian StyleShum, Hang Chee Erin, Ke Wu, Jaydutt Vadgama, and Yong Wu. 2023. "Potential Therapies Targeting the Metabolic Reprogramming of Diabetes-Associated Breast Cancer" Journal of Personalized Medicine 13, no. 1: 157. https://doi.org/10.3390/jpm13010157