Cardiac Rehabilitation for Older Women with Heart Failure
Abstract
:1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Outcomes
2.3. Statistical Analysis
2.3.1. Composite Outcome
2.3.2. Three-Year Mortality
2.3.3. Functional Outcome
3. Results
3.1. Composite Outcome
3.2. Three-Year All-Cause Mortality
3.3. Functional Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef]
- Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues about Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar]
- Scrutinio, D.; Guida, P.; Passantino, A.; Scalvini, S.; Bussotti, M.; Forni, G.; Vaninetti, R.; La Rovere, M.T. Characteristics, Outcomes, and Long-Term Survival of Patients with Heart Failure Undergoing Inpatient Cardiac Rehabilitation. Arch. Phys. Med. Rehabil. 2021, 103, 891–898.e4. [Google Scholar] [CrossRef]
- Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.; Dalal, H.; Rees, K.; Singh, S.J.; Taylor, R.S. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 2019, 1, CD003331. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, K.; Sato, Y.; Takahashi, T.; Tsuchihashi-Makaya, M.; Kotooka, N.; Ikegame, T.; Takura, T.; Yamamoto, T.; Nagayama, M.; Goto, Y.; et al. Multidisciplinary Cardiac Rehabilitation and Long-Term Prognosis in Patients with Heart Failure. Circ. Heart Fail. 2020, 13, e006798. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Maessen, M.F.H.; Bakker, E.A.; Meindersma, E.P.; Van Gorp, N.; Pijnenburg, N.; Thompson, P.D.; Hopman, M.T.E. Association of Cardiac Rehabilitation with All-Cause Mortality among Patients with Cardiovascular Disease in the Netherlands. JAMA Netw. Open 2020, 3, e2011686. [Google Scholar] [CrossRef]
- Scalvini, S.; Grossetti, F.; Paganoni, A.M.; La Rovere, M.T.; Pedretti, R.F.; Frigerio, M. Impact of in-hospital cardiac rehabilitation on mortality and readmissions in heart failure: A population study in Lombardy, Italy, from 2005 to 2012. Eur. J. Prev. Cardiol. 2019, 26, 808–817. [Google Scholar] [CrossRef]
- Scrutinio, D.; Guida, P.; Ruggieri, R.; Passantino, A. Prognostic value of functional capacity after transitional rehabilitation in older patients hospitalized for heart failure. J. Am. Geriatr. Soc. 2022, 70, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar]
- Pandey, A.; Keshvani, N.; Zhong, L.; Mentz, R.J.; Piña, I.L.; DeVore, A.D.; Yancy, C.; Kitzman, D.W.; Fonarow, G.C. Temporal Trends and Factors Associated with Cardiac Rehabilitation Participation among Medicare Beneficiaries with Heart Failure. J. Am. Coll. Cardiol. HF 2021, 9, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butrous, H.; Hummel, S.L. Heart Failure in Older Adults. Can. J. Cardiol. 2016, 32, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Supervía, M.; Medina-Inojosa, J.R.; Yeung, C.; Lopez-Jimenez, F.; Squires, R.W.; Pérez-Terzic, C.M.; Brewer, L.C.; Leth, S.E.; Thomas, R.J. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin. Proc. 2017, 92, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.R.; Thomas, R.J.; Bonikowske, A.R.; Hammer, S.M.; Olson, T.P. Sex Differences in Cardiac Rehabilitation Outcomes. Circ. Res. 2022, 130, 552–565. [Google Scholar] [CrossRef]
- Pedretti, R.F.E.; Fattirolli, F.; Griffo, R.; Ambrosetti, M.; Angelino, E.; Brazzo, S.; Corrà, U.; Dasseni, N.; Faggiano, P.; Favretto, G.; et al. Cardiac prevention and rehabilitation “3.0”: From acute to chronic phase. Position paper of the ltalian Association for Car-diovascular Prevention and Rehabilitation (GICR-IACPR). Monaldi Arch. Chest Dis. 2018, 88, 1004–1025. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Sheshadri, A.; Hsu, F.C.; Chen, S.H.; Jotwani, V.; Tranah, G.; Fielding, R.A.; Liu, C.K.; Ix, J.; Coca, S.G.; et al. Effect of Structured, Moderate Exercise on Kidney Function Decline in Sedentary Older Adults: An Ancillary Analysis of the LIFE Study Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.S.; Cohen, D.J.; Zhang, Z.; Uriel, N.; Sayer, G.; Lindenfeld, J.; Abraham, W.T.; Mack, M.J.; Stone, G.W.; Arnold, S.V. Defining a Clinically Important Change in 6-Minute Walk Distance in Patients with Heart Failure and Mitral Valve Disease. Circ. Heart Fail. 2021, 14, e007564. [Google Scholar] [CrossRef]
- Van der Heijden, G.J.; Donders, A.R.; Stijnen, T.; Moons, K.G. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: A clinical example. J. Clin. Epidemiol. 2006, 59, 1102–1109. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Voors, A.A.; Zwinderman, A.H. Factors Influencing the Predictive Power of Models for Predicting Mortality and/or Heart Failure Hospitalization in Patients with Heart Failure. JACC Heart Fail. 2014, 2, 429–436. [Google Scholar] [CrossRef]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodward, M.; Patel, A.; McMurray, J.; MacMahon, S. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Doherty, P.; Harrison, A.S.; Hossain, R. Determinants of walking fitness in patients with heart failure attending cardiac rehabilitation. Open Heart 2019, 6, e000866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, N.; Harrison, A.; Doherty, P. Factors influencing change in walking ability in patients with heart failure undergoing exercise-based cardiac rehabilitation. Int. J. Cardiol. 2018, 268, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.S.; Walker, S.; Smart, N.A.; Piepoli, M.F.; Warren, F.C.; Ciani, O.; Whellan, D.; O’Connor, C.; Keteyian, S.J.; Coats, A.; et al. Impact of Exercise Rehabilitation on Exercise Capacity and Quality-of-Life in Heart Failure: Individual Participant Meta-Analysis. J. Am. Coll. Cardiol. 2019, 73, 1430–1443. [Google Scholar] [CrossRef]
- Norris, C.M.; Yip, C.Y.; Nerenberg, K.A.; Clavel, M.A.; Pacheco, C.; Foulds, H.J.; Hardy, M.; Gonsalves, C.A.; Jaffer, S.; Parry, M. State of the Science in Women’s Cardiovascular Disease: A Canadian Perspective on the Influence of Sex and Gender. J. Am. Heart Assoc. 2020, 9, e015634. [Google Scholar] [CrossRef]
- Roger, V.L.; Weston, S.A.; Redfield, M.M.; Hellermann-Homan, J.P.; Killian, J.; Yawn, B.P.; Jacobsen, S. Trends in Heart Failure Incidence and Survival in a Community-Based Population. JAMA 2004, 292, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sellés, M.; Doughty, R.N.; Poppe, K.; Whalley, G.A.; Earle, N.; Tribouilloy, C.; McMurray, J.J.; Swedberg, K.; Køber, L.; Berry, C.; et al. Gender and survival in patients with heart failure: Interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur. J. Heart Fail. 2012, 14, 473–479. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, E.; Clayton, T.; McEntegart, M.B.; McMurray, J.J.; Piña, I.L.; Granger, C.B.; Ostergren, J.; Michelson, E.L.; Solomon, S.D.; Pocock, S.; et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: Results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 115, 3111–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghali, J.K.; Krause-Steinrauf, H.J.; Adams, K.F.; Khan, S.S.; Rosenberg, Y.D.; Yancy, C.W.; Young, J.B.; Goldman, S.; Peberdy, M.A.; Lindenfeld, J. Gender differences in advanced heart failure: Insights from the BEST study. J. Am. Coll. Cardiol. 2003, 42, 2128–2134. [Google Scholar] [CrossRef]
- Stolfo, D.; Uijl, A.; Vedin, O.; Strömberg, A.; Faxén, U.L.; Rosano, G.M.C.; Sinagra, G.; Dahlström, U.; Savarese, G. Sex-Based Differences in Heart Failure Across the Ejection Fraction Spectrum: Phenotyping, and Prognostic and Therapeutic Implications. J. Am. Coll. Cardiol. HF 2019, 7, 505–515. [Google Scholar]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [Green Version]
- Piña, I.L.; Bittner, V.; Clare, R.M.; Swank, A.; Kao, A.; Safford, R.; Nigam, A.; Barnard, D.; Walsh, M.N.; Ellis, S.J.; et al. Effects of exercise training on outcomes in women with heart failure: Analysis of HF-ACTION (Heart Failure-A Controlled Trial Investigating Outcomes of Exercise TraiNing) by sex. J. Am. Coll. Cardiol. HF 2014, 2, 180–186. [Google Scholar]
- Suaya, J.A.; Stason, W.B.; Ades, P.A.; Normand, S.-L.T.; Shepard, D.S. Cardiac Rehabilitation and Survival in Older Coronary Patients. J. Am. Coll. Cardiol. 2009, 54, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Kalogeropoulos, A.P.; Fonarow, G.C.; Georgiopoulou, V.; Burkman, G.; Siwamogsatham, S.; Patel, A.; Li, S.; Papadimitriou, L.; Butler, J. Characteristics and Outcomes of Adult Outpatients with Heart Failure and Improved or Recovered Ejection Fraction. JAMA Cardiol. 2016, 1, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, A.; Fine, N.; Ezekowitz, J.A.; Howlett, J.; Youngson, E.; McAlister, F.A. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: An echocardiogram-based registry study. Eur. Heart J. 2019, 40, 2110–2117. [Google Scholar] [CrossRef]
- Subramaniam, A.V.; Weston, S.A.; Killian, J.M.; Schulte, P.J.; Roger, V.L.; Redfield, M.M.; Blecker, S.B.; Dunlay, S.M. Development of Advanced Heart Failure: A Population-Based Study. Circ. Heart Fail. 2022, 15, e009218. [Google Scholar] [CrossRef]
- Forman, D.E.; Arena, R.; Boxer, R.; Dolansky, M.A.; Eng, J.J.; Fleg, J.L.; Haykowsky, M.; Jahangir, A.; Kaminsky, L.A.; Kitzman, D.W.; et al. Prioritizing Functional Capacity as a Principal End Point for Therapies Oriented to Older Adults with Cardiovascular Disease: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2017, 135, e894–e918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.P.; Duarte, K.; Graves, T.L.; Zile, M.; Abraham, W.T.; Weaver, F.A.; Lindenfeld, J.; Zannad, F. Natriuretic Peptides, 6-Min Walk Test, and Quality-of-Life Questionnaires as Clinically Meaningful Endpoints in HF Trials. J. Am. Coll. Cardiol. 2016, 68, 2690–2707. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, C.; Ellis, R.P.; Eyssel, F.; Zou, J.; Schiebinger, L. Sex and gender analysis improves science and engineering. Nature 2019, 575, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Guida, P.; Passantino, A.; Scalvini, S.; Bussotti, M.; Forni, G.; Tibollo, V.; Vaninetti, R.; La Rovere, M.T. Association of improvement in functional capacity after rehabilitation with long-term survival in heart failure. Int. J. Cardiol. 2022, 352, 92–97. [Google Scholar] [CrossRef]


| Females (n = 878) | Males (n = 1467) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 79 (7) | 76 (7) | <0.001 |
| Age > 75 years, n (%) | 624 (71.1) | 786 (53.6) | <0.001 |
| Comorbidities | |||
| Obesity (body mass index ≥ 30), n (%) | 188 (21.4) | 311 (21.2) | 0.903 |
| Hypertension, n (%) | 727 (82.8) | 942 (64.2) | <0.001 |
| Diabetes mellitus, n (%) | 266 (30.4) | 519 (35.5) | 0.012 |
| Chronic obstructive pulmonary disease, n (%) | 197 (22.4) | 447 (30.5) | <0.001 |
| Chronic kidney disease, n (%) | 646 (74.3) | 984 (67.1) | <0.001 |
| Stage 3a (eGFR 45–59 mL/min/1.73 m2) | 188 (21.6) | 373 (25.4) | <0.001 |
| Stage 3b (eGFR 30–44 mL/min/1.73 m2) | 253 (29.1) | 385 (26.3) | |
| Stage 4 (eGFR 15–29 mL/min/1.73 m2) | 180 (20.7) | 207 (14.1) | |
| Stage 5 (eGFR < 15 mL/min/1.73 m2) | 25 (2.9) | 19 (1.3) | |
| Anemia (hemoglobin < 13 g/dL in men and <12 g/dL in women), n (%) | 485 (56.0) | 855 (58.5) | 0.243 |
| Atrial fibrillation, n (%) | 463 (52.9) | 653 (44.5) | <0.001 |
| Clinical findings | |||
| Transferred from acute care hospitals after a hospitalization for HF, n (%) | 418 (47.6) | 672 (45.8) | 0.398 |
| NYHA III/IV class, n (%) | 474 (54) | 822 (56.0) | 0.334 |
| ICD/CRT-D, n (%) | 79 (9.0) | 354 (24.1) | <0.001 |
| ICD/CRT-D in patients with LVEF ≤ 0.40, n (%) | 64 (20.7) | 317 (31.5) | <0.001 |
| Systolic blood pressure (mm Hg), mean (SD) | 117 (17) | 113 (17) | <0.001 |
| Systolic blood pressure < 100 mm Hg, n (%) | 80 (9.6) | 227 (16.2) | <0.001 |
| Diastolic blood pressure (mm Hg), mean (SD) | 69 (9) | 68 (9) | 0.009 |
| Left ventricular ejection fraction | |||
| Mean (SD) | 47 (14) | 36 (13) | <0.001 |
| ≥0.50, n (%) | 443 (50.5) | 287 (19.6) | <0.001 |
| 0.41–0.49, n (%) | 126 (14.4) | 173 (11.8) | |
| ≤0.40, n (%) | 309 (35.2) | 1007 (68.6) | |
| Laboratory findings | |||
| Hemoglobin (g/dL), mean (SD) | 11.6 (1.8) | 12.4 (2.0) | <0.001 |
| Creatinine (mg/dL), (mean (SD) | 1.35 (0.63) | 1.58 (0.74) | <0.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 47 (22) | 52 (23) | <0.001 |
| Sodium (mEq/L), mean (%) | 139.4 (3.7) | 139.0 (3.8) | 0.015 |
| Sodium < 136 mEq/L, n (%) | 116 (13.4) | 235 (16.0) | 0.080 |
| NT-proBNP (pg/mL), median (IQR) | 2985 (1052–5988) | 2995 (1162–6104) | 0.257 |
| Functional status | |||
| Barthel index at admission, mean (SD) | 69 (26) | 74 (26) | <0.001 |
| Barthel index at admission ≤ 60 (total/severe dependence), n (%) | 231 (35.5) | 276 (25.8) | <0.001 |
| Six-min walking distance at admission (meters), mean (SD) | 137 (132) | 224 (158) | <0.001 |
| Length of stay in the IRFs (days), mean (SD) | 22 (16) | 21 (11) | 0.001 |
| Evidence-based treatments (patients with LVEF ≤ 0.40 discharged home) | |||
| Number of patients | 284 | 899 | |
| RAAS-Is, n (%) | 220 (77.5) | 708 (78.8) | 0.645 |
| Beta-blockers | 248 (87.3) | 822 (91.4) | 0.039 |
| RAAS-Is plus beta-blockers | 197 (69.4) | 656 (73.0) | 0.237 |
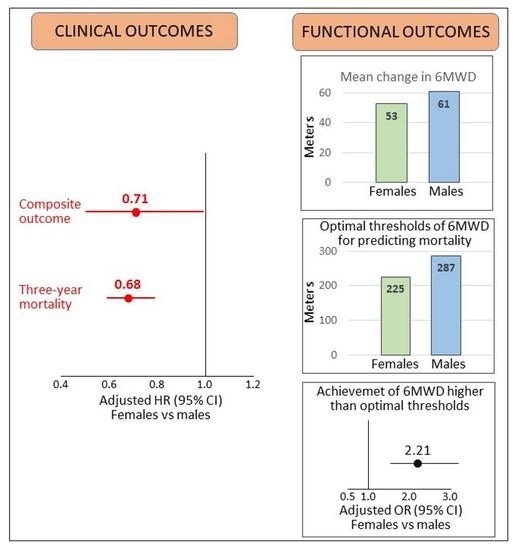
| Outcomes | HR (95% CI) Females vs. Males | p Value |
|---|---|---|
| Composite outcome | 0.71 (0.50–1.00) | 0.049 |
| Three-year mortality | 0.68 (0.59–0.79) | <0.001 |
| Functional outcome | OR (95% CI) Females vs. males | |
| Increase in 6MWD to the highest quartile of change from admission to discharge in the overall cohort | 0.80 (0.62–1.05) | 0.117 |
| Increase in 6MWD at discharge to values higher than the optimal threshold to predict mortality | 2.21 (1.53–3.20) | 0.001 |
| Patients with available data for NT-proBNP | ||
| HR (95% CI) Females vs. males | ||
| Composite outcome | 0.58 (0.36–0.93) | 0.023 |
| Three-year mortality | 0.65 (0.53–0.79) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scrutinio, D.; Guida, P.; Dalla Vecchia, L.A.; Corrà, U.; Passantino, A. Cardiac Rehabilitation for Older Women with Heart Failure. J. Pers. Med. 2022, 12, 1980. https://doi.org/10.3390/jpm12121980
Scrutinio D, Guida P, Dalla Vecchia LA, Corrà U, Passantino A. Cardiac Rehabilitation for Older Women with Heart Failure. Journal of Personalized Medicine. 2022; 12(12):1980. https://doi.org/10.3390/jpm12121980
Chicago/Turabian StyleScrutinio, Domenico, Pietro Guida, Laura Adelaide Dalla Vecchia, Ugo Corrà, and Andrea Passantino. 2022. "Cardiac Rehabilitation for Older Women with Heart Failure" Journal of Personalized Medicine 12, no. 12: 1980. https://doi.org/10.3390/jpm12121980