Ultrasound Placental Remodeling Patterns and Pathology Characteristics in Patients with History of Mild SARS-CoV-2 Infection during Pregnancy
Abstract
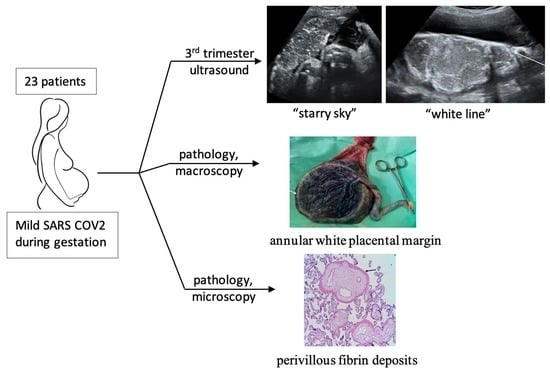
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Image Analysis
2.3. Placental Pathology
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Ultrasound Findings Tailored Accord to the Timing of the SARS-CoV-2 Infection
3.2. Pathology Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Hessami, K.; Homayoon, N.; Hashemi, A.; Vafaei, H.; Kasraeian, M.; Asadi, N. COVID-19 and maternal, fetal and neonatal mortality: A systematic review. J. Matern. Fetal Neonatal Med. 2022, 35, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Babarinsa, I.A.; Okunoye, G.O.; Odukoya, O. Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-1) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in pregnancy—An overview. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 171–175. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Han, X.; Ye, J.; Li, R. Potential Effect of COVID-19 on Maternal and Infant Outcome: Lesson from SARS. Front. Pediatr. 2020, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Graham, A.L. Potential Maternal and Infant Outcomes from Coronavirus 2019-nCoV (SARS-CoV-2) Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency from COVID-19 Causes Stillbirth and Neonatal Death from Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Prochaska, E.; Jang, M.; Burd, I. COVID-19 in pregnancy: Placental and neonatal involvement. Am. J. Reprod. Immunol. 2020, 84, e13306. [Google Scholar] [CrossRef]
- Bouachba, A.; Allias, F.; Nadaud, B.; Massardier, J.; Mekki, Y.; Duchamp, M.B.; Fourniere, B.D.; Huissoud, C.; Trecourt, A.; Collardeau-Frachon, S. Placental lesions and SARS-CoV-2 infection: Diffuse placenta damage associated to poor fetal outcome. Placenta 2021, 112, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kouba, I.; Bracero, L.; Millington, K.; Blitz, M.J. Placental calcifications after coronavirus disease 2019 in first trimester of pregnancy: Ultrasound and pathology findings. Med. Ultrason. 2022. [Google Scholar] [CrossRef]
- Soto-Torres, E.; Hernandez-Andrade, E.; Huntley, E.; Mendez-Figueroa, H.; Blackwell, S.C. Ultrasound and Doppler findings in pregnant women with SARS-CoV-2 infection. Ultrasound Obstet. Gynecol. 2021, 58, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Sotiriadis, A.; Chaoui, R.; Costa, F.; D’Antonio, F.; Heath, P.T.; Jones, C.; Malinger, G.; Odibo, A.; Prefumo, F.; et al. ISUOG Practice Guidelines: Role of ultrasound in congenital infection. Ultrasound Obstet. Gynecol. 2020, 56, 128–151. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.; Tharmaratnam, S.; Mahsud, S.; Dornan, J. Ultrasonic evidence of placental calcification at 36 weeks’ gestation: Maternal and fetal outcomes. Acta Obstet. Gynecol. Scand. 2005, 84, 7–10. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [Green Version]
- Dombrowski, M.P.; Berry, S.M.; Johnson, M.P.; Saleh, A.A.; Sokol, R.J. Birth weight-length ratios, ponderal indexes, placental weights, and birth weight-placenta ratios in a large population. Arch. Pediatr. Adolesc. Med. 1994, 148, 508–512. [Google Scholar] [CrossRef]
- David, L.C. Statistics in Medicine; Little, Brown: Boston, MA, USA, 1974. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Ob-servational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet Lond Engl. 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, A.B.; Rubin, C.S.; Cooper, H.S.; Nisenbaum, H.L.; Cole-Beuglet, C.; Medoff, J.; Goldberg, B.B. Ultrasound findings in hepatitis. Radiology 1980, 136, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.G.; Ghulmiyyah, L.M.; Tamim, H.M.; Makki, M.; Jeha, D.; Nassar, A.H. To Ignore or Not to Ignore Placental Calcifications on Prenatal Ultrasound: A Systematic Review and Meta-analysis. J. Matern. Neonatal Med. 2018, 31, 797–804. [Google Scholar] [CrossRef]
- Verteramo, R.; Santi, E.; Ravennati, F.; Scutiero, G.; Greco, P.; Morano, D. Ultrasound Findings of Fetal Infections: Current Knowledge. Reprod. Med. 2022, 3, 201–221. [Google Scholar] [CrossRef]
- Rathbun, K.M.; Hildebrand, J.P. Placenta Abnormalities; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459355/ (accessed on 21 January 2023).
- Dumont, S.; Balduyck, J.; Reynders, M.; Vanwalleghem, L.; Lebbe, B. Acute SARS-CoV-2 alpha variant infection leading to placental insufficiency and fetal distress. J. Med. Virol. 2022, 94, 1196–1200. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. from SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leeuw, A.J.M.; Luttikhuis, M.A.M.; Wellen, A.C.; Müller, C.; Calkhoven, C.F. Obesity and its impact on COVID-19. J. Mol. Med. Berl. Ger. 2021, 99, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.-C.; Krücken, I.; Hiller, G.G.R.; Niedermair, M.; Perac, K.; Pietsch, C.; Höhn, A.K. Placental pathology in sudden intrauterine death (SIUD) in SARS-CoV-2-positive oligosymptomatic women. Arch. Gynecol. Obstet. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Mulkey, S.B.; Roberts, D.J. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: Clinical–pathologic correlations. Am. J. Obstet. Gynecol. 2022, 228, 261–269. [Google Scholar] [CrossRef]




| Characteristic | Reference Group (n = 23) |
|---|---|
| BMI categories, no. (%) | |
| Underweight | 1 (4) |
| Normal weight | 14 (61) |
| Overweight | 6 (26) |
| Obese | 2 (9) |
| Parity, no (%) | |
| 1 | 8 (35) |
| 2 | 8 (35) |
| >=3 | 7 (30) |
| Timing of SARS-CoV-2 infection, no. (%) | |
| 1st trimester | 6 (26.1) |
| 2nd trimester | 15 (65.2) |
| 3rd trimester | 2 (8.7) |
| Birth weight (g), arithmetic mean ± standard deviation | |
| 1st trimester SARS-CoV-2 infection | 3391.67 ± 272.79 |
| 2nd trimester SARS-CoV-2 infection | 3660 ± 370.42 |
| 3rd trimester SARS-CoV-2 infection | 3975 ± n/a |
| Characteristics | Whole Group (n = 23) | First Trimester (n = 6) | Second Trimester (n = 15) | Third Trimester (n = 2) |
|---|---|---|---|---|
| WG at first mention of placental changes, Mean ± st. dev. | 34.75 ± 2.42 | 35.8 ± 1.1 | 34.33 ± 3.01 | 32 ± n/a |
| WG from infection to first mention of ultrasound placental changes, median (25th–75th percentile) | 19.5 (7.5; 30) | 30 (30; 32) | 9.5 (7; 16) | - |
| Amniotic fluid index, no. (%) | ||||
| 9 (39.1) 12 (52.2) 2 (8.7) | 4 (66.7) 2 (33.3) 0 (0) | 5 (33.3) 8 (53.3) 2 (13.3) | 0 (0) 2 (100) 0 (0) |
| Continuous hyperechoic line | ||||
| Data from the first evaluator, no. (%) | 14 (60.9) | 4 (66.7) | 9 (60) | 1 (50) |
| Reproducibility score, median (25th–75th percentile) | 0.50 (0.13; 1.00) | 0.63 (0.00; 1.00) | 0.50 (0.13; 1.00) | 0.63 (0.25; 1.00) |
| Placental hyperechoic areas | ||||
| Data from the first evaluator, no (%) | ||||
| -absent | 12 (52.2) | 3 (50) | 8 (53.3) | 1 (50) |
| -focal | 2 (8.7) | 0 (0) | 2 (13.3) | 0 (0) |
| -diffuse | 9 (39.1) | 3 (50) | 5 (33.3) | 1 (50) |
| Reproducibility score, median (25th–75th percentile) | 1.00 (0.75; 1.63) | 1.00 (0.5; 1.75) | 1.25 (1; 1.38) | 1.25 (0.5; 2) |
| Characteristics | Whole Group (n = 23) | First Trimester Infection (n = 6) | Second Trimester Infection (n = 15) | Third Trimester Infection (n = 2) |
|---|---|---|---|---|
| Placental weight(g), mean ± st. dev. | 529.04 ± 77.2 | 491.33 ± 39.81 | 542.87 ± 84.86 | 538.5 ± n/a |
| Feto-placental ratio, mean ± st. dev. | 6.91 ± 0.78 | 6.92 ± 0.63 | 6.84 ± 0.87 | 7.4 ± n/a |
| Maternal vascular malperfusion lesions, no. (%) | ||||
| Distal Villous Hypoplasia | 20 (87 | 5 (83.3) | 14 (93.3) | 1 (50) |
| Accelerated villous maturation | 14 (63.6) | 5 (83.3) | 8 (53.3) | 1 (50) |
| Fetal vascular malperfusion, no. (%) | 9 (39.1) | 0 (0) | 8 (53.3) | 1 (50) |
| Overall perivillous fibrin depositions, no. (%) | 18 (78.3) | 4 (66.7) | 13 (86.7) | 1 (50) |
| Absent | 5 (21.7) | 2 (33.3) | 8 (13.3) | 1 (50) |
| Normal subchorionic | 14 (60.9) | 3 (50) | 10 (66.7) | 0 (0) |
| Massive | 4 (17.4) | 1 (16.7) | 3 (20) | 1 (50) |
| Intervillous fibrin deposits, no. (%) | 9 (39.1) | 4 (66.7) | 5 (33.3) | 0 (0) |
| Stem villi perivillous fibrin deposits, no. (%) | 20 (87) | 4 (66.7) | 14 (93.3) | 2 (100) |
| None | 3 (13) | 2 (33.3) | 1 (6.7) | 0 (0) |
| Focal | 15 (65.2) | 3 (50) | 11 (73.3) | 1 (50) |
| Frequent | 5 (21.7) | 1 (16.7) | 3 (20) | 1 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staicu, A.; Albu, C.; Nemeti, G.; Bondor, C.I.; Boitor-Borza, D.; Preda, A.P.; Florian, A.; Goidescu, I.G.; Sachelaru, D.; Bora, N.; et al. Ultrasound Placental Remodeling Patterns and Pathology Characteristics in Patients with History of Mild SARS-CoV-2 Infection during Pregnancy. Diagnostics 2023, 13, 1200. https://doi.org/10.3390/diagnostics13061200
Staicu A, Albu C, Nemeti G, Bondor CI, Boitor-Borza D, Preda AP, Florian A, Goidescu IG, Sachelaru D, Bora N, et al. Ultrasound Placental Remodeling Patterns and Pathology Characteristics in Patients with History of Mild SARS-CoV-2 Infection during Pregnancy. Diagnostics. 2023; 13(6):1200. https://doi.org/10.3390/diagnostics13061200
Chicago/Turabian StyleStaicu, Adelina, Camelia Albu, Georgiana Nemeti, Cosmina Ioana Bondor, Dan Boitor-Borza, Andreia Paraschiva Preda, Andreea Florian, Iulian Gabriel Goidescu, Diana Sachelaru, Nelida Bora, and et al. 2023. "Ultrasound Placental Remodeling Patterns and Pathology Characteristics in Patients with History of Mild SARS-CoV-2 Infection during Pregnancy" Diagnostics 13, no. 6: 1200. https://doi.org/10.3390/diagnostics13061200