Biochemical and Epigenetic Modulations under Drought: Remembering the Stress Tolerance Mechanism in Rice
Abstract
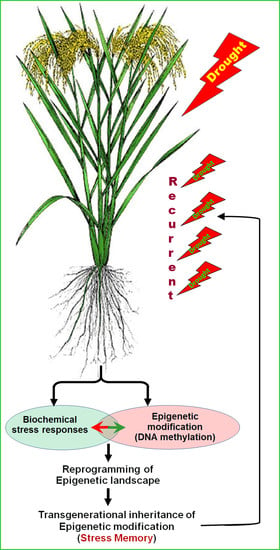
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Stress Imposition
2.2. Estimation of Soil Moisture and Relative Water Content
2.3. Estimation of Chlorophyll Content
2.4. Estimation of Lipid Peroxidation
2.5. Estimation of Antioxidant Activity
2.6. Estimation of Total Phenolics Content
2.7. Estimation of Proline Content
2.8. Estimation of Genome-Wide 5-Methylcytosine Content
2.9. Statistical Analysis
3. Results
3.1. Effect of Drought Stress/Lowered Soil Moisture Content on Relative Water Content of Leaf
3.2. Effect of Terminal Drought Stress on Chlorophyll Content
3.3. Effect of Drought Stress on Lipid Peroxidation in Leaf
3.4. Effect of Drought Stress on Antioxidant Potential in Leaves of Different Rice Genotypes
3.5. Change in 5-Methylcytosine (5-mC) Content on Drought Stress in Rice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fantao, Z.; Yuan, L.; Meng, Z.; Yi, Z.; Hongping, C.; Biaolin, H.; Jiankun, X. Identification and characterization of drought stress-responsive novel microRNAs in Dongxiang wild rice. Rice Sci. 2018, 25, 175–184. [Google Scholar] [CrossRef]
- Finatto, T.; de Oliveira, A.C.; Chaparro, C.; da Maia, L.C.; Farias, D.R.; Woyann, L.G.; Picault, N. Abiotic stress and genome dynamics: Specific genes and transposable elements response to iron excess in rice. Rice 2015, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. The role of biopesticides in sustainably feeding the nine billion global populations. J. Biofertil. Biopestici. 2013, 4, e114. [Google Scholar]
- Kumar, S.; Kumar, S.; Krishnan, S.G.; Mohapatra, T. Molecular basis of genetic plasticity to varying environmental conditions on growing rice by dry/direct-sowing and exposure to drought stress: Insights for DSR varietal development. Front. Plant Sci. 2022, 13, 1013207. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]
- Kumar, S. Saving water for ecological integrity: Agricultural perspective of Per Drop More Crop. Int. J. En Sci. Nat. Resour. 2021, 28, 1–7. [Google Scholar]
- Kumar, S. Epigenetics and epigenomics for crop improvement: Current opinion. Adv. Biotechnol. Microbiol. 2019, 14, 555879. [Google Scholar]
- Kumar, S.; Mohapatra, T. Dynamics of DNA methylation and its functions in plant growth and development. Front. Plant Sci. 2021, 12, 596236. [Google Scholar] [CrossRef]
- Vergara, Z.; Gutierrez, C. Emerging roles of chromatin in the maintenance of genome organization and function in plants. Genome Biol. 2017, 18, 96. [Google Scholar] [CrossRef]
- Kumar, S. Epigenetic memory of stress responses in plants. J. Phytochem. Biochem. 2018, 2, e102. [Google Scholar]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Liu, B.; Zhou, H.; Elmongy, M.S.; Xia, Y. Combined proteome and transcriptome analysis of heat-primed azalea reveals new insights into plant heat acclimation memory. Front. Plant Sci. 2020, 11, 1278. [Google Scholar] [CrossRef]
- Sasi, M.; Awana, M.; Samota, M.K.; Tyagi, A.; Kumar, S.; Sathee, L.; Singh, A. Plant growth regulator induced mitigation of oxidative burst helps in the management of drought stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 185, 104413. [Google Scholar] [CrossRef]
- Kumar, S.; Beena, A.S.; Awana, M.; Singh, A. Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front. Plant Sci. 2017, 8, 1151. [Google Scholar] [CrossRef]
- Kumar, S.; Seem, K.; Kumar, S.; Mohapatra, T. RNA-seq analysis reveals the genes/pathways responsible for genetic plasticity of rice to varying environmental conditions on direct-sowing and transplanting. Sci. Rep. 2022, 12, 2241. [Google Scholar] [CrossRef]
- Zheng, Y.; Xia, Z.; Wu, J.; Ma, H. Effects of repeated drought stress on the physiological characteristics and lipid metabolism of Bombax ceiba L. during subsequent drought and heat stresses. BMC Plant Biol. 2017, 21, 467. [Google Scholar] [CrossRef]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Schulze, W.X.; Altenbuchinger, M.; He, M.; Kränzlein, M.; Zörb, C. Proteome profiling of repeated drought stress reveals genotype-specific responses and memory effects in maize. Plant Physiol. Biochem. 2021, 159, 67–79. [Google Scholar] [CrossRef]
- Caballero, J.I.; Verduzco, C.V.; Galan, J.; Jimenez, E.S.D. Proline accumulation as a symptom of drought stress in maize: A tissue differentiation requirement. J. Expt. Bot. 2005, 39, 889–897. [Google Scholar] [CrossRef]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Holub, P.; Nezval, J.; Štroch, M.; Špunda, M.; Urban, O.; Jansen, M.A.K.; Klem, K. Induction of phenolic compounds by UV and PAR is modulated by leaf ontogeny and barley genotype. Plant Physiol. Biochem. 2019, 134, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Kaur, S.; Seem, K.; Duhan, N.; Kumar, S.; Kaundal, R.; Mohapatra, T. Transcriptome and physio-biochemical profiling reveals differential responses of rice cultivars at reproductive-stage drought stress. Int. J. Mol. Sci. 2023, 24, 1002. [Google Scholar] [CrossRef] [PubMed]
- Trust, B.; Shin, N.; Jim, E.D.; Harry, D.S. Antioxidant activity of pearled wheat androller milled fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar]
- Rao, S.P.; Mishra, B.; Gupta, S.R.; Rathore, A. Physiological response to salinity and alkalinity of rice genitypes of varying salt tolerance grown in field lysimeters. J. Stress Physiol. Biochem. 2013, 9, 54–65. [Google Scholar]
- Singh, A.; Bhushan, B.; Gaikwad, K.; Yadav, O.P.; Kumar, S.; Rai, R.D. Induced defence responses of contrasting bread wheat genotypes under differential salt stress imposition. Ind. J. Biochem. Biophys. 2015, 52, 75–85. [Google Scholar]
- Samota, M.K.; Sasi, M.; Awana, M.; Yadav, O.P.; Amitha Mithra, S.V.; Tyagi, A.; Kumar, S.; Singh, A. Elicitor-induced biochemical and molecular manifestations to improve drought tolerance in rice (Oryza sativa L.) through seed-priming. Front. Plant Sci. 2017, 8, 934. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant and Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Kumar, S.; Beena, A.S.; Awana, M.; Singh, A. Salt-induced tissue-specific cytosine methylation downregulates expression of HKT genes in contrasting wheat (Triticum aestivum L.) genotypes. DNA Cell Biol. 2017, 36, 283–294. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Qian, W. Active DNA demethylation: Mechanism and role in plant development. Plant Cell Rep. 2018, 37, 77–85. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress Memory and the Inevitable Effects of Drought: A Physiological Perspective. Front Plant Sci. 2016, 7, 143. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Krishnan, S.G.; Mohapatra, T. Epigenetic perspective of gene×environment interaction: Adaptability of rice under varying environmental conditions grown by direct-sowing and transplanting. Front. Plant Sci. 2023, 14, 1046604. [Google Scholar]
- Meins, F.; Thomas, M. Meiotic transmission of epigenetic changes in the cell-division factor requirement of plant cells. Development 2003, 130, 6201–6208. [Google Scholar] [CrossRef]
- Iwasaki, M.; Paszkowski, J. Epigenetic memory in plants. EMBO J. 2014, 33, 1987–1998. [Google Scholar] [CrossRef]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Lange, U.C.; Schneider, R. What an epigenome remembers. BioEssays 2010, 32, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Liu, Y.; Zhao, L.; Zhang, S.; Huang, X. A novel MYB transcription factor regulates ascorbic acid synthesis and affects cold tolerance. Plant Cell Environ. 2018, 42, 832–845. [Google Scholar] [CrossRef]
- Kim, J.-M.; To, T.K.; Ishida, J.; Matsui, A.; Kimura, H.; Seki, M. Transition of chromatin status during the process of recovery from drought stress in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Vu, N.T.; Cheong, J.J. Transcriptional stress memory and transgenerational inheritance of drought tolerance in plants. Int. J. Mol. Sci. 2022, 23, 12918. [Google Scholar] [CrossRef]
- Tricker, P.J.; Gibbings, J.G.; Lopez, C.M.R.; Hadley, P.; Wilkinson, M.J. Low relative humidity triggers RNA-directed de novo DNA methylation and suppression of genes controlling stomatal development. J. Exp. Bot. 2012, 63, 3799–3813. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Luo, L. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Rolando, J.L.; Yactayo, W.; Monneveux, P.; Mares, V.; Quiroz, R. Improving potato drought tolerance through the induction of long-term water stress memory. Plant Sci. 2015, 238, 26–32. [Google Scholar] [CrossRef]
- Liu, H.; Amanda, J.A.; Jason, A.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef]
- Sultan, S.E.; Barton, K.; Wilczek, A.M. Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 2009, 90, 1831–1839. [Google Scholar] [CrossRef]
- Virlouvet, L.; Fromm, M. Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol. 2015, 205, 596–607. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef]
- Abdallah, M.B.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought priming improves subsequent more severe drought in a drought-sensitive cultivar of olive cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A.; Shahid, M. Terminal drought and seed priming improves drought tolerance in wheat. Physiol. Mol. Biol. Plants 2018, 24, 845–856. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthoferm, R.; Lamuela-Raventosm, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Seem, K.; Kumar, S. Cultivation of Rice: Evolving towards climate-smart crops for precision in resource use efficiency. MC Agric. Environ. Sci. 2021, 1, 41–49. [Google Scholar]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of Soluble Sugars in Metabolism and Sensing under Abiotic Stress. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Zhang, X.; Xu, Y.; Huang, B. Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant Cell Environ. 2018, 42, 947–958. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.; Shah, S.; Wu, X.; Shaheen, A.; Xiao, L.; Wang, S. Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. 2020, 20, 486. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Tong, Z.; He, F.; Li, X. Metabolomic analyses reveal substances that contribute to the increased freezing tolerance of alfalfa (Medicago sativa L.) after continuous water deficit. BMC Plant Biol. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. Peer J. 2020, 8, e10046. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and utilization of malondehyde in exotic pine under drought stress using near-infrared spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Balyan, S.; Kumar, M.; Mutum, R.D.; Raghuvanshi, U.; Agarwal, P.; Mathur, S.; Raghuvanshi, S. Identification of miRNA-mediated drought responsive multi-tiered regulatory network in drought tolerant rice, Nagina 22. Sci. Rep. 2017, 7, 15446. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna-Chopra, R. Drought-induced spikelet sterility is associated with an inefficient antioxidant defence in rice panicles. Physiol. Plant. 2004, 121, 462–471. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, E. Effect of chilling on antioxidant enzymes and DPPH-radical scavenging activity of high-and low-vigour cucumber seedling radicles. Plant Cell Environ. 2002, 25, 1233–1238. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Al Hassan, M.; Fuertes, M.M.; Sanchez, F.J.R.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Lekshmy, V.S.; Vijayaraghavareddy, P.; Nagashree, A.N.; Ramu, V.S.; Ramegowda, V.; Makarla, U.; Sreeman, S. Induction of acquired tolerance through gradual progression of drought is the key for maintenance of spikelet fertility and yield in rice under semi-irrigated aerobic conditions. Front. Plant Sci. 2021, 11, 632919. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef]
- Cortijo, S.; Wardenaar, R.; Colome-Tatche, M.; Gilly, A.; Etcheverry, M.; Labadie, K.; Johannes, F. Mapping the epigenetic basis of complex traits. Science 2014, 343, 1145–1148. [Google Scholar] [CrossRef]
- Kooke, R.; Johannes, F.; Wardenaar, R.; Becker, F.; Etcheverry, M.; Colot, V.; Keurentjes, J.J. Epigenetic basis of morphological variation and phenotypic plasticity in Arabidopsis thaliana. Plant Cell 2015, 27, 337–348. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, S.; Seem, K.; Kumar, S.; Mohapatra, T. Understanding 3D genome organization and its effect on transcriptional gene regulation under environmental stress in plant: A Chromatin Perspective. Front. Cell Dev. Biol. 2021, 9, 774719. [Google Scholar] [CrossRef]
- Kumar, S. Genome editing to epigenome editing: Towards unravelling the enigmas in developmental biology. Trends Develop. Biol. 2019, 12, 31–38. [Google Scholar] [CrossRef]
- Gallego-Bartolomé, J.; Gardiner, J.; Liu, W.; Papikian, A.; Ghoshal, B.; Kuo, H.Y.; Jacobsen, S.E. Targeted DNA demethylation of the Arabidopsis genome using the human TET1 catalytic domain. Proc. Natl. Acad. Sci. USA 2018, 115, E2125–E2134. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Seem, K.; Mohapatra, T. Biochemical and Epigenetic Modulations under Drought: Remembering the Stress Tolerance Mechanism in Rice. Life 2023, 13, 1156. https://doi.org/10.3390/life13051156
Kumar S, Seem K, Mohapatra T. Biochemical and Epigenetic Modulations under Drought: Remembering the Stress Tolerance Mechanism in Rice. Life. 2023; 13(5):1156. https://doi.org/10.3390/life13051156
Chicago/Turabian StyleKumar, Suresh, Karishma Seem, and Trilochan Mohapatra. 2023. "Biochemical and Epigenetic Modulations under Drought: Remembering the Stress Tolerance Mechanism in Rice" Life 13, no. 5: 1156. https://doi.org/10.3390/life13051156