Beyond the Limits of Light: An Application of Super-Resolution Confocal Microscopy (sCLSM) to Investigate Eocene Amber Microfossils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fossil Material
2.2. Preparation of Amber
2.3. Imaging of Fossils
2.4. Modern Mites
2.5. Imaging of Modern Mites
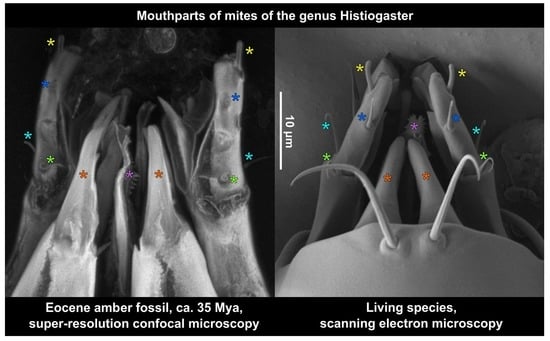
3. Results
3.1. Autofluorescence Gradient in Amber Is Correlated with Amber Deterioration (“Yellowing”)
3.2. Comparing sCLSM with Transmitted and Reflected Light Microscopy
3.3. Estimating the Resolution of sCLSM
4. Discussion
4.1. Ancient Habitat
4.2. Fluorescence of Fossils and Amber
4.3. Resolution of sCLSM in Amber Fossils Compared to Other Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, A.R.; Jancke, S.; Lindquist, E.E.; Ragazzi, E.; Roghi, G.; Nascimbene, P.C.; Schmidt, K.; Wappler, T.; Grimaldi, D.A. Arthropods in amber from the Triassic Period. Proc. Natl. Acad. Sci. USA 2012, 109, 14796–14801. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Delclòs, X.; Briggs, D.E.G.; Peñalver, E. Taphonomy of insects in carbonates and amber. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 203, 19–64. [Google Scholar] [CrossRef]
- Seyfullah, L.J.; Beimforde, C.; Dal Corso, J.; Perrichot, V.; Rikkinen, J.; Schmidt, A.R. Production and preservation of resins—Past and present. Biol. Rev. 2018, 93, 1684–1714. [Google Scholar] [CrossRef]
- Penney, D. Sub/fossil resin research in the 21st Century: Trends and perspectives. PalZ 2016, 90, 425–447. [Google Scholar] [CrossRef]
- Koller, B.; Schmitt, J.M.; Tischendorf, G. Cellular fine structures and histochemical reactions in the tissue of a cypress twig preserved in Baltic amber. Proc. Biol. Sci. 2005, 272, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, J.T.; Müller, C.H.G.; Sombke, A. A centipede nymph in Baltic amber and a new approach to document amber fossils. Org. Divers. Evol. 2013, 13, 425–432. [Google Scholar] [CrossRef]
- Olmi, M.; Eggs, B.; Capradossi, L.; van de Kamp, T.; Perkovsky, E.E.; Guglielmino, A.; Vasilenko, D.V. A new species of Bocchus from upper Eocene Rovno amber (Hymenoptera, Dryinidae). J. Hymenopt. Res. 2022, 92, 257–272. [Google Scholar] [CrossRef]
- Baranov, V.; Hoffeins, C.; Hoffeins, H.-W.; Haug, J.T. Reaching across the ocean of time: A midge morphotype from the Cretaceous of Gondwana found in the Eocene Baltic amber. Palaeontol. Electron. 2019, 22, 1–17. [Google Scholar] [CrossRef]
- Koch, C.L.; Berendt, G.C. Die im Bernstein befindlichen Crustaceen, Myriopoden, Arachniden und Apteren der Vorwelt. In Die in Bernstein Befindlichen Organischen Reste der Vorwelt, Gesammelt, in Verbindung Mit Mehreren Bearbeitetet und Herausgegeben; Berendt, G.C., Ed.; Commission der Nicolaischen Buchhandlung: Berlin, Germany, 1854; Volume 1, pp. 1–124. [Google Scholar]
- Sidorchuk, E.A. New Technique for Preparation of Small-Sized Amber Samples with Application to Mites. In Insect Evolution in an Amberiferous and Stone Alphabet, Proceedings of the 6th International Congress on Fossil Insects, Arthropods and Amber, Byblos, Lebanon, 14–18 April 2013; Azar, D., Engel, M.S., Jarzembowski, E., Krogmann, L., Nel, A., Santiago-Blay, J., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2013; pp. 189–201. [Google Scholar]
- Sidorchuk, E.A.; Vorontsov, D.D. Preparation of small-sized 3D amber samples: State of the technique. Palaeoentomology 2018, 1, 80–90. [Google Scholar] [CrossRef]
- Khaustov, A.A.; Vorontsov, D.D.; Perkovsky, E.E.; Lindquist, E.E. Review of fossil heterostigmatic mites (Acari: Heterostigmata) from late Eocene Rovno Amber. I. Families Tarsocheylidae, Dolichocybidae and Acarophenacidae. Syst. Appl. Acarol. 2021, 26, 33–61. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Bonwich, E.; Delannoy, M.; Doberstein, S. Electron microscopic studies of mummified tissues in amber fossils. Am. Mus. Novit. 1994, 3097, 1–31. [Google Scholar]
- Sidorchuk, E.A.; Norton, R.A. Redescription of the fossil oribatid mite Scutoribates perornatus, with implications for systematics of Unduloribatidae (Acari: Oribatida). Zootaxa 2010, 2666, 45–67. [Google Scholar] [CrossRef]
- Speranza, M.; Wierzchos, J.; Alonso, J.; Bettucci, L.; Martín-González, A.; Ascaso, C. Traditional and New Microscopy Techniques Applied to the Study of Microscopic Fungi Included in Amber. In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1135–1145. [Google Scholar]
- Saint Martin, J.-P.; Saint Martin, S. Exquisite preservation of a widespread filamentous microorganism in French Cretaceous ambers: Crucial for revising a controversial fossil. C. R. Palevol 2018, 17, 415–434. [Google Scholar] [CrossRef]
- Sadowski, E.-M.; Hofmann, C.-C. The largest amber-preserved flower revisited. Sci. Rep. 2023, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Soriano, C.; Archer, M.; Azar, D.; Creaser, P.; Delclòs, X.; Godthelp, H.; Hand, S.; Jones, A.; Nel, A.; Néraudeau, D.; et al. Synchrotron X-ray imaging of inclusions in amber. C. R. Palevol 2010, 9, 361–368. [Google Scholar] [CrossRef]
- Nadein, K.S.; Perkovsky, E.E.; Moseyko, A.G. New Late Eocene Chrysomelidae (Insecta: Coleoptera) from Baltic, Rovno and Danish ambers. Pap. Palaeontol. 2016, 2, 117–137. [Google Scholar] [CrossRef]
- Perreau, M.; Haelewaters, D.; Tafforeau, P. A parasitic coevolution since the Miocene revealed by phase-contrast synchrotron X-ray microtomography and the study of natural history collections. Sci. Rep. 2021, 11, 2672. [Google Scholar] [CrossRef]
- Lak, M.; Néraudeau, D.; Nel, A.; Cloetens, P.; Perrichot, V.; Tafforeau, P. Phase Contrast X-ray Synchrotron Imaging: Opening Access to Fossil Inclusions in Opaque Amber. Microsc. Microanal. 2008, 14, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.A.; Engel, M.S.; Hammel, J.; Hörnig, M.; van de Kamp, T.; Zuber, M.; Haug, J.T. Synchrotron-radiation computed tomography uncovers ecosystem functions of fly larvae in an Eocene forest. Palaeontol. Electron. 2021, 24, 1–22. [Google Scholar] [CrossRef]
- Wang, H.; Matzke-Karasz, R.; Horne, D.J. Mid-Cretaceous coastal amber forest palaeoenvironment revealed by exceptionally preserved ostracods from an extant lineage. Foss. Rec. 2022, 25, 147–172. [Google Scholar] [CrossRef]
- Heethoff, M.; Norton, R.A. A new use for synchrotron X-ray microtomography: Three-dimensional biomechanical modeling of chelicerate mouthparts and calculation of theoretical biteforces. Invertebr. Biol. 2009, 128, 332–339. [Google Scholar] [CrossRef]
- Heethoff, M.; Helfen, L.; Norton, R.A. Description of Neoliodes dominicus n. sp. (Acari, Oribatida) from Dominican Amber, Aided by Synchrotron X-ray Microtomography. J. Paleontol. 2009, 83, 153–159. [Google Scholar] [CrossRef]
- Müller, D.; Graetz, J.; Balles, A.; Stier, S.; Hanke, R.; Fella, C. Laboratory-Based Nano-Computed Tomography and Examples of Its Application in the Field of Materials Research. Crystals 2021, 11, 677. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Wirth, S.; Penney, D.; McNeil, A.; Bradley, R.S.; Withers, P.J.; Preziosi, R.F. A minute fossil phoretic mite recovered by phase-contrast X-ray computed tomography. Biol. Lett. 2011, 8, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Robin, N.; Béthoux, O.; Sidorchuk, E.; Cui, Y.; Li, Y.; Germain, D.; King, A.; Berenguer, F.; Ren, D. A Carboniferous mite on an insect reveals the antiquity of an inconspicuous interaction. Curr. Biol. 2016, 26, 1376–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirejtshuk, A.G.; Chetverikov, P.E.; Azar, D. Libanopsinae, new subfamily of the family Sphindidae (Coleoptera, Cucujoidea) from Lower Cretaceous Lebanese amber, with remarks on using confocal microscopy for the study of amber inclusions. Cretac. Res. 2015, 52, 461–479. [Google Scholar] [CrossRef]
- Ball, A.D.; Goral, T.; Kamanli, S.A. Confocal microscopy applied to paleontological specimens. Paleontol. Soc. Pap. 2017, 22, 39–55. [Google Scholar] [CrossRef]
- Saint Martin, J.-P.; Saint Martin, S.; Bolte, S.; Néraudeau, D. Spider web in Late Cretaceous French amber (Vendée): The contribution of 3D image microscopy. C. R. Palevol 2014, 13, 463–472. [Google Scholar] [CrossRef]
- Speranza, M.; Ascaso, C.; Delclòs, X.; Peñalver, E. Cretaceous mycelia preserving fungal polysaccharides: Taphonomic and paleoecological potential of microorganisms preserved in fossil resins. Geol. Acta 2015, 13, 363–385. [Google Scholar] [CrossRef]
- Fu, Y.-Z.; Li, Y.-D.; Su, Y.-T.; Cai, C.-Y.; Huang, D.-Y. Application of confocal laser scanning microscopy to the study of amber bioinclusions. Palaeoentomology 2021, 4, 266–278. [Google Scholar] [CrossRef]
- Vorontsov, D.; Voronezhskaya, E.E. Pushing the limits of optical resolution in the study of the tiniest fossil arthropods. Hist. Biol. 2022, 34, 2415–2423. [Google Scholar] [CrossRef]
- Huff, J.; Bergter, A.; Birkenbeil, J.; Kleppe, I.; Engelmann, R.; Krzic, U. The new 2D Superresolution mode for ZEISS Airyscan. Nat. Methods 2017, 14, 1223. [Google Scholar] [CrossRef]
- Pastorelli, G.; Shashoua, Y.; Richter, J. Surface yellowing and fragmentation as warning signs of depolymerisation in Baltic amber. Polym. Degrad. Stab. 2013, 98, 2317–2322. [Google Scholar] [CrossRef]
- Mitov, P.G.; Perkovsky, E.E.; Dunlop, J.A. Harvestmen (Arachnida: Opiliones) in Eocene Rovno amber (Ukraine). Zootaxa 2021, 4984, 43–72. [Google Scholar] [CrossRef]
- Michels, J.; Gorb, S.N. Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J. Microsc. 2012, 245, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rabasović, M.D.; Pantelić, D.V.; Jelenković, B.M.; Ćurčić, S.B.; Rabasović, M.S.; Vrbica, M.D.; Lazović, V.M.; Ćurčić, B.P.M.; Krmpot, A.J. Nonlinear microscopy of chitin and chitinous structures: A case study of two cave-dwelling insects. J. Biomed. Opt. 2015, 20, 016010. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Scolari, F. Autofluorescent Biomolecules in Diptera: From Structure to Metabolism and Behavior. Molecules 2022, 27, 4458. [Google Scholar] [CrossRef]
- Bolton, S.J.; Klompen, H.; Bauchan, G.R.; Ochoa, R. A new genus and species of Nematalycidae (Acari: Endeostigmata). J. Nat. Hist. 2014, 48, 1359–1373. [Google Scholar] [CrossRef]
- Huff, J. The Airyscan Detector: Confocal Microscopy Evolution for the Neurosciences. In Advanced Optical Methods for Brain Imaging; Kao, F.-J., Keiser, G., Gogoi, A., Eds.; Progress in Optical Science and Photonics; Springer: Singapore, 2019; pp. 83–102. ISBN 978-981-10-9020-2. [Google Scholar]
- Zachvatkin, A. Arachnoidea. Tyroglyphoidea. In Fauna of USSR; English Translation 1959; American Institute of Biological Science: Washington, DC, USA, 1941; Volume 6, pp. 1–474. [Google Scholar]
- Woodring, J. North American Tyroglyphidae (Acari): III. The genus Histiogaster, with description of four new species. Proc. Lousiana Acad. Sci. 1966, 29, 113–136. [Google Scholar]
- OConnor, B.; University of Michigan, Museum of Zoology, Ann Arbor, MI, USA. Personal communication, 2022.
- Ascaso, C.; Wierzchos, J.; Speranza, M.; Gutiérrez, J.C.; González, A.M.; de los Ríos, A.; Alonso, J. Fossil protists and fungi in amber and rock substrates. Micropaleontology 2005, 51, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Lozano, R.P.; Pérez-de la Fuente, R.; Barrón, E.; Rodrigo, A.; Viejo, J.L.; Peñalver, E. Phloem sap in Cretaceous ambers as abundant double emulsions preserving organic and inorganic residues. Sci. Rep. 2020, 10, 9751. [Google Scholar] [CrossRef] [PubMed]
- Bisulca, C.; Nascimbene, P.C.; Elkin, L.; Grimaldi, D.A. Variation in the deterioration of fossil resins and implications for the conservation of fossils in amber. Am. Mus. Novit. 2012, 3734, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sadowski, E.-M.; Schmidt, A.R.; Seyfullah, L.J.; Solórzano-Kraemer, M.M.; Neumann, C.; Perrichot, V.; Hamann, C.; Milke, R.; Nascimbene, P.C. Conservation, preparation and imaging of diverse ambers and their inclusions. Earth-Sci. Rev. 2021, 220, 103653. [Google Scholar] [CrossRef]
- Klaus, A.V.; Kulasekera, V.L.; Schawaroch, V. Three-dimensional visualization of insect morphology using confocal laser scanning microscopy. J. Microsc. 2003, 212, 107–121. [Google Scholar] [CrossRef]
- Chetverikov, P. Confocal laser scanning microscopy technique for the study of internal genitalia and external morphology of eriophyoid mites (Acari: Eriophyoidea). Zootaxa 2012, 3453, 56–68. [Google Scholar] [CrossRef]
- Cómbita-Heredia, O.; Gulbronson, C.J.; Ochoa, R.; Quintero-Gutiérrez, E.J.; Bauchan, G.; Klompen, H. Size, shape, and direction matters: Matching secondary genital structures in male and female mites using multiple microscopy techniques and 3D modeling. PLoS ONE 2021, 16, e0254974. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L.; Mans, B.J.; Handschuh, S.; Dunlop, J.A. A remarkable assemblage of ticks from mid-Cretaceous Burmese amber. Parasitology 2022, 149, 820–830. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorontsov, D.D.; Kolesnikov, V.B.; Voronezhskaya, E.E.; Perkovsky, E.E.; Berto, M.M.; Mowery, J.; Ochoa, R.; Klimov, P.B. Beyond the Limits of Light: An Application of Super-Resolution Confocal Microscopy (sCLSM) to Investigate Eocene Amber Microfossils. Life 2023, 13, 865. https://doi.org/10.3390/life13040865
Vorontsov DD, Kolesnikov VB, Voronezhskaya EE, Perkovsky EE, Berto MM, Mowery J, Ochoa R, Klimov PB. Beyond the Limits of Light: An Application of Super-Resolution Confocal Microscopy (sCLSM) to Investigate Eocene Amber Microfossils. Life. 2023; 13(4):865. https://doi.org/10.3390/life13040865
Chicago/Turabian StyleVorontsov, Dmitry D., Vasiliy B. Kolesnikov, Elena E. Voronezhskaya, Evgeny E. Perkovsky, Marielle M. Berto, Joseph Mowery, Ronald Ochoa, and Pavel B. Klimov. 2023. "Beyond the Limits of Light: An Application of Super-Resolution Confocal Microscopy (sCLSM) to Investigate Eocene Amber Microfossils" Life 13, no. 4: 865. https://doi.org/10.3390/life13040865