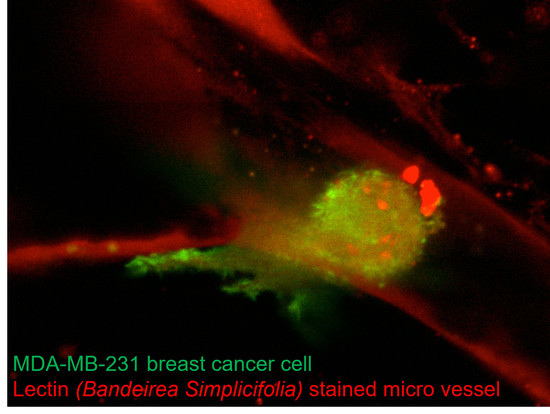
Lectin Staining of Microvascular Glycocalyx in Microfluidic Cancer Cell Extravasation Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microfluidic Device Design, Fabrication, and Cell Culture
2.2. General Cell Culture and Cell Expansion
2.3. Cancer Cell Extravasation Assay
2.4. Immunocytochemistry and Phalloidine Staining
2.5. Confocal Imaging
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reitsma, S.; Slaaf, D.W.; Vink, H.; Van Zandvoort, M.A.M.J.; Egbrink, M.G.A.O. The endothelial glycocalyx: Composition, functions, and visualization. Pflügers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offeddu, G.; Hajal, C.; Foley, C.; Wan, Z.; Ibrahim, L.; Coughlin, M.; Kamm, R. Glycocalyx-Mediated Vascular Dissemination of Circulating Tumor Cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mensah, S.A.; Harding, I.C.; Zhang, M.; Jaeggli, M.P.; Torchilin, V.P.; Niedre, M.J.; Ebong, E.E. Metastatic cancer cell attachment to endothelium is promoted by endothelial glycocalyx sialic acid degradation. AIChE J. 2019, 65, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Astapenko, D.; Ticha, A.; Tomasova, A.; Hyspler, R.; Zadak, Z.; Lehmann, C.; Cerny, V. Evaluation of endothelial glycocalyx in healthy volunteers—An observational study. Clin. Hemorheol. Microcirc. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.; Patterson, A.M.; Gardner, L.; Schmutz, C.; Ashton, B.A.; Botella, L.M.; Sánchez-Elsner, T.; Sanz-Rodriguez, F.; Kojima, S.; Shimada, J.; et al. Leukocyte extravasation: Chemokine transport and presentation by the endothelium. Blood 2002, 100, 3853–3860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.; Nejadhamzeeigilani, Z.; Gutowski, N.J.; Whatmore, J.L. Loss of the endothelial glycocalyx is associated with increased E-selectin mediated adhesion of lung tumour cells to the brain microvascular endothelium. J. Exp. Clin. Cancer Res. 2015, 34, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Follain, G.; Osmani, N.; Azevedo, A.S.; Allio, G.; Mercier, L.; Karreman, M.A.; Solecki, G.; Leòn, M.J.G.; Lefebvre, O.; Fekonja, N.; et al. Hemodynamic Forces Tune the Arrest, Adhesion, and Extravasation of Circulating Tumor Cells. Dev. Cell 2018, 45, 33–52.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.B.; Whisler, J.A.; Jeon, J.S.; Kamm, R.D. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 2013, 5, 1262–1271. [Google Scholar] [CrossRef] [Green Version]
- Mensah, S.A.; Nersesyan, A.A.; Ebong, E.E. Endothelial Glycocalyx-Mediated Intercellular Interactions: Mechanisms and Implications for Atherosclerosis and Cancer Metastasis. Cardiovasc. Eng. Technol. 2020, 1–19. [Google Scholar] [CrossRef]
- Ilina, O.; Campanello, L.; Gritsenko, P.G.; Vullings, M.; Wang, C.; Bult, P.; Losert, W.; Friedl, P. Intravital microscopy of collective invasion plasticity in breast cancer. Dis. Model. Mech. 2018, 11, dmm034330. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Valadez, A.V.; Zuo, P.; Nie, Z. Microfluidic 3D cell culture: Potential application for tissue-based bioassays. Bioanalysis 2012, 4, 1509–1525. [Google Scholar] [CrossRef] [Green Version]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosadegh, B.; Huang, C.; Park, J.W.; Shin, H.S.; Chung, B.G.; Hwang, S.-K.; Lee, K.-H.; Kim, H.J.; Brody, J.; Jeon, N.L. Generation of Stable Complex Gradients Across Two-Dimensional Surfaces and Three-Dimensional Gels. Langmuir 2007, 23, 10910–10912. [Google Scholar] [CrossRef] [PubMed]
- Kamm, R.D.; Chung, S.; Vickerman-Kelley, V.V. Three-Dimensional Microfluidic Platforms and Methods of Use Therof. U.S. Patent 9,121,847 B2, 1 December 2015. [Google Scholar]
- Ho, Y.T.; Adriani, G.; Beyer, S.; Nhan, P.-T.; Kamm, R.D.; Kah, J.C.Y. A Facile Method to Probe the Vascular Permeability of Nanoparticles in Nanomedicine Applications. Sci. Rep. 2017, 7, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, S.; Schürmann, R.; Feldmann, I.; Blocki, A.; Bald, I.; Schneider, R.; Emmerling, F. Maintaining Stable Zeolitic Imidazolate Framework (ZIF) Templates during Polyelectrolyte Multilayer Coating. Colloid Interface Sci. Commun. 2018, 22, 14–17. [Google Scholar] [CrossRef]
- Kantak, C.; Zhu, Q.; Beyer, S.; Bansal, T.; Trau, D. Utilizing microfluidics to synthesize polyethylene glycol microbeads for Förster resonance energy transfer based glucose sensing. Biomicrofluidics 2012, 6, 022006–220069. [Google Scholar] [CrossRef] [Green Version]
- Whisler, J.A.; Chen, M.B.; Kamm, R.D. Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System. Tissue Eng. Part C Methods 2014, 20, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Petroll, W.M. Cell Motility and Mechanics in Three-Dimensional Collagen Matrices. Annu. Rev. Cell Dev. Biol. 2010, 26, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Blocki, A.; Beyer, S.; Dewavrin, J.-Y.; Goralczyk, A.; Wang, Y.; Peh, P.; Yingting, W.; Moonshi, S.S.; Vuddagiri, S.; Raghunath, M.; et al. Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long-term retention of MSCs in infarcted myocardium. Biomaterials 2015, 53, 12–24. [Google Scholar] [CrossRef]
- Assunção, M.; Wong, C.W.; Richardson, J.J.; Tsang, R.; Beyer, S.; Raghunath, M.; Blocki, A. Macromolecular dextran sulfate facilitates extracellular matrix deposition by electrostatic interaction independent from a macromolecular crowding effect. Mater. Sci. Eng. C 2020, 106, 110280. [Google Scholar] [CrossRef]
- Pan, H.M.; Beyer, S.; Zhu, Q.; Trau, D. Inwards Interweaving of Polymeric Layers within Hydrogels: Assembly of Spherical Multi-Shells with Discrete Porosity Differences. Adv. Funct. Mater. 2013, 23, 5108–5115. [Google Scholar] [CrossRef]
- Beyer, S.; Koch, M.; Lee, Y.H.; Jung, F.; Blocki, A. An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase. Int. J. Mol. Sci. 2018, 19, 2913. [Google Scholar] [CrossRef] [Green Version]
- Mack, P.J.; Zhang, Y.; Chung, S.; Vickerman, V.; Kamm, R.D.; García-Cardenña, G. Biomechanical Regulation of Endothelium-dependent Events Critical for Adaptive Remodeling. J. Biol. Chem. 2009, 284, 8412–8420. [Google Scholar] [CrossRef] [Green Version]
- Morin, K.T.; Tranquillo, R.T. In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp. Cell Res. 2013, 319, 2409–2417. [Google Scholar] [CrossRef] [Green Version]
- Blocki, A.; Beyer, S.; Jung, F.; Raghunath, M. The controversial origin of pericytes during angiogenesis—Implications for cell-based therapeutic angiogenesis and cell-based therapies. Clin. Hemorheol. Microcirc. 2018, 69, 215–232. [Google Scholar] [CrossRef] [Green Version]
- Lukasz, A.; Hillgruber, C.; Oberleithner, H.; Kusche-Vihrog, K.; Pavenstädt, H.; Rovas, A.; Hesse, B.; Goerge, T.; Kümpers, P. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc. Res. 2017, 113, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Moskowitz, M.; Sims, J. Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int. J. Mol. Med. 2007, 19. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Z.; Yang, F.; Huang, Y.; Yu, Y.; Zhou, L.; Sun, Z.; Cui, D.; Yan, Y. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing by Enhancing Angiogenesis through Delivering Angiopoietin-2. Stem Cell Rev. Rep. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bersini, S.; Miermont, A.; Pavesi, A.; Kamm, R.D.; Thiery, J.P.; Moretti, M.; Adriani, G. A combined microfluidic-transcriptomic approach to characterize the extravasation potential of cancer cells. Oncotarget 2018, 9, 36110–36125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vallejo, J.J.; Van Dijk, W.; Hof, B.V.H.; Van Die, I.; Engelse, M.A.; Van Hinsbergh, V.W.; Gringhuis, S.I. Activation of human endothelial cells by tumor necrosis factor-α results in profound changes in the expression of glycosylation-related genes. J. Cell. Physiol. 2005, 206, 203–210. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Hamil, T.; Creighton, J.; Wu, S.; Bhat, P.; McDonald, F.; Stevens, T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc. Res. 2004, 67, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Augustin-Voss, H.G.; Smith, C.A.; Lewis, R.M. Phenotypic Characterization of Normal and Neoplastic Canine Endothelial Cells by Lectin Histochemistry. Veter. Pathol. 1990, 27, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, S.; Blocki, A.; Cheung, M.C.Y.; Wan, Z.H.Y.; Mehrjou, B.; Kamm, R.D. Lectin Staining of Microvascular Glycocalyx in Microfluidic Cancer Cell Extravasation Assays. Life 2021, 11, 179. https://doi.org/10.3390/life11030179
Beyer S, Blocki A, Cheung MCY, Wan ZHY, Mehrjou B, Kamm RD. Lectin Staining of Microvascular Glycocalyx in Microfluidic Cancer Cell Extravasation Assays. Life. 2021; 11(3):179. https://doi.org/10.3390/life11030179
Chicago/Turabian StyleBeyer, Sebastian, Anna Blocki, Matthew Chung Yin Cheung, Zoe Ho Ying Wan, Babak Mehrjou, and Roger Dale Kamm. 2021. "Lectin Staining of Microvascular Glycocalyx in Microfluidic Cancer Cell Extravasation Assays" Life 11, no. 3: 179. https://doi.org/10.3390/life11030179