New Insights in the Ontogeny and Taphonomy of the Devonian Acanthodian Triazeugacanthus affinis From the Miguasha Fossil-Lagerstätte, Eastern Canada
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials




2.2. Scanning Electron Microscopy (SEM) Observation and Energy Dispersive X-Ray Spectrometry (EDX) Analysis
2.3. Fourier Transform Infrared Spectrometry
2.4. X-Ray Diffraction
3. Results
3.1. SEM Observations and EDX Analyses of Skeletal Tissues
3.1.1. Fossilized Tissues of Triazeugacanthus affinis



3.1.2. Living Models: Centroscyllium fabricii and Scomber scombrus
3.2. Mineralogical Analysis of Triazeugacanthus Skeleton

4. Discussion
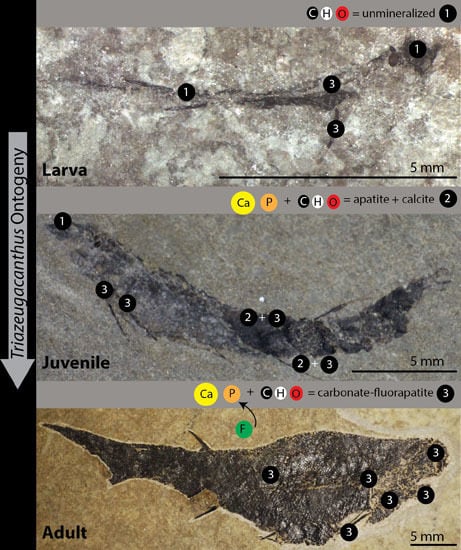
4.1. Chemical Composition of Triazeugacanthus Biomineralized Tissues Depends on Ontogenetic Stages
4.2. Preservation versus Recrystallization of Triazeugacanthus Tissues
5. Concluding Remarks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cloutier, R. The fossil record of fish ontogenies: Insights into developmental patterns and processes. Semin. Cell Dev. Biol. 2010, 21, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, P.C.J.; Sansom, I.J. Origin and early evolution of vertebrate skeletonization. Microsc. Res. Tech. 2002, 59, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Kemp, N.E.; Westrin, S.K. Ultrastructure of calcified cartilage in the endoskeletal tesserae of sharks. J. Morphol. 1979, 160, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.N.; Summers, A.P. Mineralized cartilage in the skeleton of chondrichthyan fishes. Zoology 2006, 109, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Grünbaum, T.; Cloutier, R.; Vincent, B. Dynamic skeletogenesis in fishes: Insight of exercise training on developmental plasticity. Dev. Dyn. 2012, 241, 1507–1524. [Google Scholar] [CrossRef] [PubMed]
- Faustino, M.; Power, D.M. Osteologic development of the viscerocranial skeleton in sea bream: Alternative ossification strategies in teleost fish. J. Fish Biol. 2001, 58, 537–572. [Google Scholar] [CrossRef]
- Gavaia, P.J.; Sarasquete, C.; Cancela, M.L. Detection of mineralized structures in early stages of development of marine Teleostei using a modified alcian blue-alizarin red double staining technique for bone and cartilage. Biotech. Histochem. 2000, 75, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Johanson, Z.; Trinajstic, K. Fossilized ontogenies: The contribution of placoderm ontogeny to our understanding of the evolution of early gnathostomes. Palaeontology 2014, 57, 505–516. [Google Scholar] [CrossRef]
- Trueman, C.N.; Privat, K.; Field, J. Why do crystallinity values fail to predict the extent of diagenetic alteration of bone mineral? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 160–167. [Google Scholar] [CrossRef]
- Morris, S.C.; Caron, J.-B. Pikaia gracilens Walcott, a stem-group chordate from the Middle Cambrian of British Columbia. Biol. Rev. 2012, 87, 480–512. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, M.; Desbiens, S.; Janvier, P.; Kerr, J. New data on the soft tissues and external morphology of the antiarch Bothriolepis canadensis (Whiteaves, 1880), from the Upper Devonian of Miguasha, Quebec. In Recent Advances in the Origin and Early Radiation of Vertebrates; Arratia, G., Wilson, M.V.H., Cloutier, R., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2004; pp. 439–454. [Google Scholar]
- Trinajstic, K.; Marshall, C.; Long, J.; Bifield, K. Exceptional preservation of nerve and muscle tissues in Late Devonian placoderm fish and their evolutionary implications. Biol. Lett. 2007, 3, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, R. Great Canadian Lagerstätten 4. The Devonian Miguasha biota (Québec): UNESCO World Heritage Site and a time capsule in the early history of vertebrates. Geosci. Can. 2013, 40, 149–163. [Google Scholar] [CrossRef]
- Kolodny, Y.; Luz, B.; Sander, M.; Clemens, W.A. Dinosaur bones: Fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 126, 161–171. [Google Scholar] [CrossRef]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [PubMed]
- Cambra-Moo, O.; Nacarino-Meneses, C.; Díaz-Güemes, I.; Enciso, S.; Gil, O.G.; Rodríguez, L.L.; Barbero, M.Á.R.; Antonio, H.; Martín, A.G. Multidisciplinary characterization of the long-bone cortex growth patterns through sheep’s ontogeny. J. Struct. Biol. 2015, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Allison, P.A.; Briggs, D.E.G. Taphonomy of Nonmineralized Tissues. In Taphonomy: Releasing the Data Locked in the Fossil Record; Allison, P.A., Briggs, D.E.G., Eds.; Plenum Press: New York, NY, USA, 1991; Volume 9. [Google Scholar]
- Schopf, J.M. Modes of fossil preservation. Rev. Palaeobot. Palynol. 1975, 20, 27–53. [Google Scholar] [CrossRef]
- Briggs, D.E.G. The role of decay and mineralization in the preservation of soft-bodied fossils. Annu. Rev. Earth Planet. Sci. 2003, 31, 275–301. [Google Scholar] [CrossRef]
- Cloutier, R.; Béchard, I.; Charest, F.; Matton, O. La contribution des poissons fossiles du parc national de Miguasha à la biologsie évolutive du développement. Nat. Can. 2009, 133, 84–95. (In French) [Google Scholar]
- Chevrinais, M.; Cloutier, R.; Sire, J.-Y. The revival of a so-called rotten fish: The ontogeny of the Devonian acanthodian Triazeugacanthus. Biol. Lett. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, R.; Proust, J.-N.; Tessier, B. The Miguasha Fossil-Fish-Lagerstätte: A consequence of the Devonian land-sea interactions. Palaeobiodivers. Palaeoenviron. 2011, 91, 293–323. [Google Scholar] [CrossRef]
- Gupta, N.S.; Cambra-Moo, O.; Briggs, D.E.G.; Love, G.D.; Fregenal-Martinez, M.A.; Summons, R.E. Molecular taphonomy of macrofossils from the Cretaceous Las Hoyas Formation, Spain. Cretac. Res. 2008, 29, 1–8. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Avci, R.; Collier, T.; Goodwin, M.B. Microscopic, chemical and molecular methods for examining fossil preservation. Comptes Rendus Palevol 2008, 7, 159–184. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Shemesh, A. Crystallinity and diagenesis of sedimentary apatites. Geochim. Cosmochim. Acta 1990, 54, 2433–2438. [Google Scholar] [CrossRef]
- Schultze, H.-P. A new acanthodian from the Pennsylvanian of Utah, USA, and the distribution of otoliths in gnathostomes. J. Vertebr. Paleontol. 1990, 10, 49–58. [Google Scholar] [CrossRef]
- Yi, H.; Balan, E.; Gervais, C.; Ségalen, L.; Roche, D.; Person, A.; Fayon, F.; Morin, G.; Babonneau, F. Probing atomic scale transformation of fossil dental enamel using Fourier transform infrared and nuclear magnetic resonance spectroscopy: A case study from the Tugen Hills (Rift Gregory, Kenya). Acta Biomater. 2014, 10, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Pucéat, E.; Reynard, B.; Lécuyer, C. Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem. Geol. 2004, 205, 83–97. [Google Scholar] [CrossRef]
- Balan, E.; Delattre, S.; Roche, D.; Segalen, L.; Morin, G.; Guillaumet, M.; Blanchard, M.; Lazzeri, M.; Brouder, C.; Salje, E.K.H. Line-broadening effects in the powder infrared spectrum of apatite. Phys. Chem. Miner. 2011, 38, 111–122. [Google Scholar] [CrossRef]
- MacFadden, B.J.; Labs-Hochstein, J.; Quitmyer, I.; Jones, D.S. Incremental growth and diagenesis of skeletal parts of the lamnoid shark Otodus obliquus from the early Eocene (Ypresian) of Morocco. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 206, 179–192. [Google Scholar] [CrossRef]
- Labs-Hochstein, J.; MacFadden, B.J. Quantification of diagenesis in Cenozoic sharks: Elemental and mineralogical changes. Geochim. Cosmochim. Acta 2006, 70, 4921–4932. [Google Scholar] [CrossRef]
- Trueman, C.N.G.; Behrensmeyer, A.K.; Tuross, N.; Weiner, S. Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: Diagenetic mechanisms and the role of sediment pore fluids. J. Archaeol. Sci. 2004, 31, 721–739. [Google Scholar] [CrossRef]
- Yi, H.; Balan, E.; Gervais, C.; Segalen, L.; Fayon, F.; Roche, D.; Person, A.; Morin, G.; Guillaumet, M.; Blanchard, M. A carbonate-fluoride defect model for carbonate-rich fluorapatite. Am. Mineral. 2013, 98, 1066–1069. [Google Scholar] [CrossRef]
- El Albani, A.; Cloutier, R.; Candilier, A.-M. Early diagenesis of the Upper Devonian Escuminac Formation in the Gaspé Peninsula, Québec: Sedimentological and geochemical evidence. Sediment. Geol. 2002, 146, 209–223. [Google Scholar] [CrossRef]
- Matton, O.; Cloutier, R.; Stevenson, R. Apatite for destruction: Isotopic and geochemical analyses of bioapatites and sediments from the Upper Devonian Escuminac Formation (Miguasha, Québec). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 361, 73–83. [Google Scholar] [CrossRef]
- Béland, P.; Arsenault, M. Scauménellisation de l’Acanthodii Triazeugacanthus affinis (Whiteaves) de la Formation d'Escuminac (Dévonien supérieur de Miguasha, Québec): Révision du Scaumenella mesacanthi Graham-Smith. Can. J. Earth Sci. 1985, 22, 514–524. (In French) [Google Scholar]
- Young, R.A.; Brown, W.E. Structures of Biological Minerals. In Biological Mineralization and Demineralization; Springer: Berlin, Germany, 1982; pp. 101–141. [Google Scholar]
- Graham-Smith, W. Scaumenella mesacanthi, gen. et sp. n., a peculiar organism from the Upper Devonian of Scaumenac Bay, Province Quebec, Canada. Ann. Mag. Nat. Hist. 1935, 16, 473–476. [Google Scholar] [CrossRef]
- Gagnier, P.-Y. Acanthodii. In Devonian Fishes and Plants of Miguasha, Quebec, Canada; Schultze, H.-P., Cloutier, R., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 1996; pp. 149–164. [Google Scholar]
- Franz-Odendaal, T.A.; Vickaryous, M.K. Skeletal elements in the vertebrate eye and adnexa: Morphological and developmental perspectives. Dev. Dyn. 2006, 235, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Burrow, C.J.; Newman, M.J.; Davidson, R.G.; Den Blaauwen, J.L. Sclerotic plates or circumorbital bones in early jawed fishes? Palaeontology 2011, 54, 207–214. [Google Scholar] [CrossRef]
- Brazeau, M.D. The braincase and jaws of a Devonian “acanthodian” and modern gnathostome origins. Nature 2009, 457, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Finarelli, J.A.; Coates, M.I. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature 2012, 486, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, G.; Parker, A.R.; Hasegawa, Y.; Siveter, D.J.; Yamamoto, R.; Miyashita, K.; Takahashi, Y.; Ito, S.; Wakamatsu, K.; Mukuda, T. Mineralized rods and cones suggest colour vision in a 300 Myr-old fossil fish. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.S. The vertebrate eye. In Functional Vertebrate Morphology; Hildebrand, M., Bramble, D.M., Liem, K.F., Wake, D.B., Eds.; Harvard University Press: Cambridge, MA, USA, 1985; pp. 317–337. [Google Scholar]
- Goodenough, D.A. The crystalline lens. A system networked by gap junctional intercellular communication. Semin. Cell Biol. 1992, 3, 49–58. [Google Scholar] [CrossRef]
- Chidiac, Y. Paleoenvironmental interpretation of the Escuminac Formation based on geochemical evidence. In Devonian Fishes and Plants of Miguasha, Quebec, Canada; Schultze, H.P., Cloutier, R., Eds.; Verlag Dr Friedrich Pfeil: München, Germany, 1996; pp. 47–53. [Google Scholar]
- Sanchez, S.; Dupret, V.; Tafforeau, P.; Trinajstic, K.M.; Ryll, B.; Gouttenoire, P.-J.; Wretman, L.; Zylberberg, L.; Peyrin, F.; Ahlberg, P.E. 3D microstructural architecture of muscle attachments in extant and fossil vertebrates revealed by synchrotron microtomography. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Tafforeau, P.; Ahlberg, P.E. The humerus of Eusthenopteron: A puzzling organization presaging the establishment of tetrapod limb bone marrow. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.P.; Donoghue, P.C.J. Skeletal histology of Bothriolepis canadensis (Placodermi, Antiarchi) and evolution of the skeleton at the origin of jawed vertebrates. J. Morphol. 2009, 270, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Nemliher, J.G.; Baturin, G.N.; Kallaste, T.E.; Murdmaa, I.O. Transformation of hydroxyapatite of bone phosphate from the ocean bottom during fossilization. Lithol. Miner. Resour. 2004, 39, 468–479. [Google Scholar] [CrossRef]
- Wang, Y.; Von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.-M.; et al. Water-mediated structuring of bone apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Kalvoda, J.; Novák, M.; Bábek, O.; Brzobohatý, R.; Holá, M.; Holoubek, I.; Kanický, V.; Škoda, R. Compositional changes in fish scale hydroxylapatite during early diagenesis; an example from an abandoned meander. Biogeochemistry 2009, 94, 197–215. [Google Scholar] [CrossRef]
- Sansom, R.S.; Gabbott, S.E.; Purnell, M.A. Atlas of vertebrate decay: A visual and taphonomic guide to fossil interpretation. Palaeontology 2013, 56, 457–474. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Ding, D.Y. Experimental fluoridation of nanocrystalline apatite. Am. Mineral. 2009, 94, 53–63. [Google Scholar] [CrossRef]
- Cosmidis, J.; Benzerara, K.; Menguy, N.; Arning, E. Microscopy evidence of bacterial microfossils in phosphorite crusts of the Peruvian shelf: Implications for phosphogenesis mechanisms. Chem. Geol. 2013, 359, 10–22. [Google Scholar] [CrossRef]
- Cosmidis, J.; Benzerara, K.; Gheerbrant, E.; Estève, I.; Bouya, B.; Amaghzaz, M. Nanometer-scale characterization of exceptionally preserved bacterial fossils in Paleocene phosphorites from Ouled Abdoun (Morocco). Geobiology 2013, 11, 139–153. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Harper, D.B. Fluorine-containing natural products. J. Fluor. Chem. 1999, 100, 127–133. [Google Scholar] [CrossRef]
- Murphy, C.D.; Schaffrath, C.; O’Hagan, D. Fluorinated natural products: The biosynthesis of fluoroacetate and 4-fluorothreonine in Streptomyces cattleya. Chemosphere 2003, 52, 455–461. [Google Scholar] [CrossRef]
- Ifrim, C.; Stinnesbeck, W.; Frey, E. Upper Cretaceous (Cenomanian-Turonian and Turonian-Coniacian) open marine plattenkalk deposits in NE Mexico. Neues Jahrb. Geol. Paläontol. Abh. 2007, 245, 71–81. [Google Scholar] [CrossRef]
- Rude, P.D.; Aller, R.C. Fluorine mobility during early diagenesis of carbonate sediment: An indicator of mineral transformations. Geochim. Cosmochim. Acta 1991, 55, 2491–2509. [Google Scholar] [CrossRef]
- Allison, P.A. The role of anoxia in the decay and mineralization of proteinaceous macro-fossils. Paleobiology 1988, 14, 139–154. [Google Scholar]
- Zylberberg, L.; Meunier, F.J.; Laurin, M. A microanatomical and histological study of the postcranial dermal skeleton in the Devonian sarcopterygian Eusthenopteron foordi. Acta Palaeontol. Pol. 2010, 55, 459–470. [Google Scholar] [CrossRef]
- Reynard, B.; Balter, V. Trace elements and their isotopes in bones and teeth: Diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 4–16. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevrinais, M.; Balan, E.; Cloutier, R. New Insights in the Ontogeny and Taphonomy of the Devonian Acanthodian Triazeugacanthus affinis From the Miguasha Fossil-Lagerstätte, Eastern Canada. Minerals 2016, 6, 1. https://doi.org/10.3390/min6010001
Chevrinais M, Balan E, Cloutier R. New Insights in the Ontogeny and Taphonomy of the Devonian Acanthodian Triazeugacanthus affinis From the Miguasha Fossil-Lagerstätte, Eastern Canada. Minerals. 2016; 6(1):1. https://doi.org/10.3390/min6010001
Chicago/Turabian StyleChevrinais, Marion, Etienne Balan, and Richard Cloutier. 2016. "New Insights in the Ontogeny and Taphonomy of the Devonian Acanthodian Triazeugacanthus affinis From the Miguasha Fossil-Lagerstätte, Eastern Canada" Minerals 6, no. 1: 1. https://doi.org/10.3390/min6010001