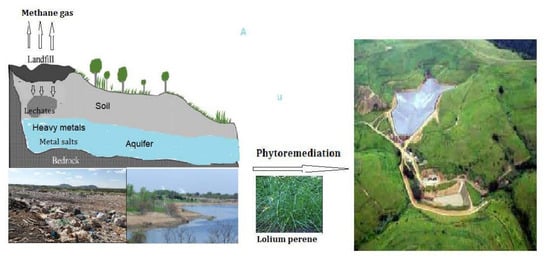
Extraction Potential of Lolium perenne L. (Perennial Rye Grass) for Metals in Landfill Soil: Its Tolerance and Defense Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pot Trial
2.2. pH and Conductivity
2.3. Metal Analysis
2.3.1. Acid-Assisted Microwave Digestion of Samples
2.3.2. Determination of the Concentration of Metals in Soil, Leaves and Roots
2.3.3. Analysis of a Certified Reference Material
2.4. Enzyme Extraction and Assay
2.4.1. Total Amylolytic Activity (TAA)
2.4.2. Glutathione S-Transferase Activity (GST)
2.4.3. Peroxidase Activity (POD)
2.4.4. Superoxide Dismutase Activity (SOD)
2.5. Scanning Electron Microscopy
2.6. Statistical Data Analysis
3. Results and Discussion
3.1. pH and Electrical Conductivity of the Soil
3.2. Plant Growth and Metal Uptake
Validation of the Analytical Method
3.3. Metal Uptake from Landfill Soil
3.4. Phytoremediation Potential of L. perenne
3.5. Scanning Electron Microscopy
3.6. Enzyme Activity
3.6.1. Superoxide Dismutase Activity
3.6.2. Glutathione Transferase Activity
3.6.3. Peroxidase Activity
3.6.4. Total Amylolytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.R.; Owens, G. Potential for enhanced phytoremediation of landfills using biosolids—A review. J. Environ. Manag. 2010, 91, 791–797. [Google Scholar] [CrossRef] [PubMed]
- The World Bank Annual Report. Available online: https://thedocs.worldbank.org/en/doc/596391540568499043-0340022018/original/worldbankannualreport2016.pdf (accessed on 28 September 2016).
- Faiza, M.T.; Hassan, N.A.; Mohammad Farhan, R.; Edre, M.A.; Rus, R.M. Solid Waste: Its Implication for Health and Risk of Vector Borne Diseases. JWBM 2019, 1, 14–17. [Google Scholar]
- Aljaradin, M.; Persson, K.M. Municipal Landfilling Practice and its Impact on the Water Resources-Jordan. World Environ. 2014, 5, 213–218. [Google Scholar]
- Jayawardhana, Y.; Kumarathilaka, P.; Herath, I.; Vithanage, M. Municipal Solid Waste Biochar for Prevention of Pollution from Landfill Leachate. In Environmental Materials and Waste; Academic Press: Hong Kong, China, 2016; pp. 117–148. [Google Scholar]
- Bouzayani, F.; Aydi, A.; Abichou, T. Soil Contamination by Heavy Metals in Landfills: Measurements from an Unlined Leachate Storage Basin. Environ. Monit. Assess. 2014, 186, 5033–5040. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Sci. Total Environ. 2016, 563, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Purakayastha, T.J.; Viswanath, T.; Bhadraray, S.; Chhonkar, P.K.; Adhikari, P.P.; Suribabu, K. Phytoextraction of Zinc, Copper, Nickel and Lead from a Contaminated Soil by Different Species of Brassica. Int. J. Phytoremediation 2008, 10, 63–74. [Google Scholar] [CrossRef]
- Ghori, Z.; Iftikhar, H.; Bhatti, M.F.; Sharma, I.; Kazi, A.G.; Ahmad, P. Phytoextraction: The Use of Plants to Remove Heavy Metals from Soil. J. Plant Interact. 2019, 385–409. [Google Scholar]
- Balafrej, H.B.; Bogusz, D.; Triqui, Z.E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562–684. [Google Scholar]
- Zalewska, M. Response of Perennial Ryegrass (Lolium perenne L.) to Soil Contamination with Zinc. J. Elem. 2012, 17, 329–343. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Freitas, H.M.O. Hyperaccumulation in plants–Biodiversity and prospecting for phytoremediation studies. Electron. J. Biotechnol. 2003, 6, 285–305. [Google Scholar] [CrossRef]
- Casler, M.D.U.; Undersander, D.J. Identification of Temperate Pasture Grasses and Legumes. In Horse Pasture Management; Academic Press: London, UK, 2018; pp. 11–35. [Google Scholar]
- Vatansever, R.O.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, K.; Rodwell, V.; Bender, D.; Botham, K.M.; Weil, P.A.; Kennelly, P.J. Harper’s Illustrated Biochemistry; McGraw-Hill: New York, NY, USA, 2009; Volume 28, pp. 482–483. [Google Scholar]
- Milkovic, L.C.G.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and their Implications in Therapy Concepts. Cells 2019, 8, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. J. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Mokgalaka-Matlala, N.S.; Flores-Taviz, E.; Castillo-Michel, H.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity of arsenic (III) and (V) on plant growth, element uptake, and total amylolytic activity of mesquite (Prosopis Juliflora × P. Velutina). Int. J. Phytoremediation 2008, 10, 47–60. [Google Scholar] [CrossRef]
- Fuwa, H. A new method for micro determination of amylase activity by the use of amylose as substrate. J. Biochem. 1954, 41, 583–593. [Google Scholar] [CrossRef]
- Melato, F.A.; Mokgalaka, N.S.; Mccrindle, R.I. Adaptation and Detoxification Mechanisms of Vetiver Grass (Chrysopogon Zizanioides) Growing on Gold Mine Tailings. Int. J. Phytoremediation 2016, 18, 509–520. [Google Scholar] [CrossRef]
- Elbehiry, F.; Elbasiouny, H.; Ali, R.; Brevik, E.C. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water Air Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Ou, J.; Li, H.; Yan, Z.; Zhou, Y.; Bai, L.; Zhang, C.; Wang, X.; Chen, G. In situ Immobilisation of Toxic Metals in Soil using Maifan Stone and Illite/Smectite Clay. Sci. Rep. 2018, 8, 4618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk-Szabela, D.; Markiewicz, J.; Wolf, W.M. Heavy Metal Uptake by Herbs. IV. Influence of Soil pH on the Content of Heavy Metals in Valeriana officinalis L. Water Air Soil Pollut. 2015, 226, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corwin, D.L.; Yemoto, K. Measurement of Soil Salinity: Electrical Conductivity and Total Dissolved Solids. Soil Sci. Soc. Am. J. 2019, 83, 1–2. [Google Scholar] [CrossRef]
- Mehes-Smith, M.; Nkongolo, K.; Cholewa, E. Coping Mechanisms of Plants to Metal Contaminated Soil. J. Environ. Sustain. 2013, 54, 53–90. [Google Scholar]
- Hossain, M.A.; Piyatida, P.; Da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef] [Green Version]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Berríos, G.; Rodrigues-Salvador, A.; Nunes-Nesi, A.; Deppe, M.; Demanet, R.; Rengel, Z.; Alberdi, M. Physiological and Biochemical Responses to Manganese Toxicity in Ryegrass (Lolium Perenne L.) Genotypes. Plant Physiol. Biochem. 2017, 123, 89–97. [Google Scholar] [CrossRef]
- Rosas, A.; Rengel, Z.; De La Luz Mora, M. Manganese Supply and pH Influence Growth, Carboxylate Exudation and Peroxidase Activity of Ryegrass and White Clover. J. Plant Nutr. 2007, 30, 253–270. [Google Scholar] [CrossRef]
- Rollin, H.B.; Nogueira, C.M.C.A. Manganese: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier Science: San Diego, CA, USA, 2011. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Dubey, R.S. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Dong, Y.; Hu, G.; Bai, X. Effects of Exogenous Nitric Oxide on Cadmium Toxicity and Antioxidative System in Perennial Ryegrass. J. Soil Sci. Plant Nutr. 2018, 18, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Leudo, A.M.; Cruz, Y.; Montoya-Ruiz, C.; Delgado, M.D.P.; Saldarriaga, J.F. Mercury phytoremediation with Lolium perenne-Mycorrhizae in contaminated soils. Sustainability 2020, 12, 3795. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, S.; Wang, J.; Li, S.; Xie, S.; Wang, G. Phytoremediation of uranium-contaminated soil by perennial ryegrass (Lolium perenne L.) enhanced with citric acid application. Environ. Sci. Pollut. Res. 2022, 29, 33002–33012. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga, J.F.; López, J.E.; Díaz-García, L.; Montoya-Ruiz, C. Hanges in Lolium perenne L. rhizosphere microbiome during phytoremediation of Cd-and Hg-contaminated soils. Environ. Sci. Pollut. Res. 2023, 30, 49498–49511. [Google Scholar] [CrossRef]
- Radziemska, M.; Bilgin, A.; Vaverková, M. Application of Mineral-Based Amendments for Enhancing Phytostabilization in Lolium perenne L. Cultivation. CLEAN–Soil Air Water 2018, 46, 1600679. [Google Scholar] [CrossRef]
- Takarina, N.; Tjiong, G.P. Bioconcentration Factor (BCF) and Translocation Factor (TF) of Heavy Metals in Mangrove Trees of Blanakan Fish Farm. Makara J. Sci. 2017, 21, 6–20. [Google Scholar] [CrossRef]
- Waterlot, C.; Hechelski, M. Benefits of Ryegrass on Multi-Contaminated Soils Part 1: Effects of Fertilizers on Bioavailability and Accumulation of Metals. Sustainability 2019, 11, 5093. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Neumann, P.M. The spatially variable inhibition by water deficit of maize root growt correlates with altered profiles of proton flux and cell wall pH. Plant Physiol. 2004, 135, 2291–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzeslowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant 2011, 33, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.M.; Mahmood-Ur-Rahman, A.Q.; Awan, S.I. Plant Cuticular Waxes: A Review on Functions, Composition, Biosyntheses Mechanism and Transportation. Life Sci. 2015, 2, 60–67. [Google Scholar]
- Seo, P.J.; Park, C.P. Cuticular Wax Biosynthesis as a Way of Inducing Drought Resistance. Plant Signal. Behav. 2011, 6, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased Accumulation of Cuticular Wax and Expression of Lipid Transfer Protein in Response to Periodic Drying Events in Leaves of Tree Tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berwal, M.K.; Ram, C. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2018; pp. 1–4. [Google Scholar]
- Saibi, W.; Brini, F. Superoxide Dismutase (SOD) and Abiotic Stress Tolerance in Plants: An Overview. In Superoxide Dismutase; Magliozzi, S., Ed.; Nova Science Publishers, Inc.: Sfax, Tunisia, 2018; Chapter 3; pp. 101–142. [Google Scholar]
- Akbari, M.; Katam, R.; Husain, R.; Farajpour, M.; Mazzuca, S.; Mahna, N. Sodium Chloride Induced Stress Responses of Antioxidative Activities in Leaves and Roots of Pistachio Rootstock. Biomolecules 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, F.; Xu, C.; Li, B. Simple Method For Visual Detection of Glutathione S-Transferase Activity and Inhibition Using Cysteamine-Capped Gold Nanoparticles as Colorimetric Probes. Gold Bull. 2015, 48, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Erofeeva, E.A. Dependence of Guaiacol Peroxidase Activity and Lipid Peroxidation Rate in Drooping Birch (Betula pendula Roth) and Tillet (Tilia cordata Mill) Leaf on Motor Traffic Pollution Intensity. Dose-Response 2015, 13, 1559325815588510. [Google Scholar] [CrossRef] [Green Version]
- Varga, B.; Janda, T.; László, E.; Veisz, O. Influence of Abiotic Stresses on the Antioxidant Enzyme Activity of Cereals. Acta Physiol. Plant. 2011, 34, 849–858. [Google Scholar] [CrossRef]
- Jouili, H.; Bouazizi, H.; El Ferjani, E. Plant Peroxidases: Biomarkers of Metallic Stress. Acta Physiol. Plant. 2011, 33, 2075. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1990; p. 1223. [Google Scholar]
- Busi, M.V.; Gomez-Casati, D.F.; Martín, M.; Barchiesi, J.; Grisolía, M.J.; Hedín, N.; Carrillo, J.B. Starch Metabolism in Green Plants. Polysaccharides 2015, 329–376. [Google Scholar]
- Sigfridsson, K.G.; Bernát, G.; Mamedov, F.; Styring, S. Molecular Interference of Cd2+ with Photosystem II. Biochim. Biophys. Acta Bioenerg. 2004, 1659, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirjani, M. Effects of Cadmium on Wheat Growth and Some Physiological Factors. Int. J. For. Soil Eros. 2012, 2, 50–58. [Google Scholar]
- Ware, A.; Shaikh, F.; Harke, S. Detection and Comparative Analysis of Amylase Activity from Leaves, Seeds and Stem of Purslane (Portulacaoleracea). Int. J. Adv. Res. Sci. Eng. 2017, 6, 793–798. [Google Scholar]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant Enzyme Activities and Abiotic Stress Tolerance Relationship in Vegetable Crops. In Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; IntechOpen: London, UK, 2016; pp. 481–503. ISBN 978-953-51-2250-0. [Google Scholar]













| Operating Parameter | Spectro ARCO® ICP-OES | ELAN DRC-e ICP-MS |
|---|---|---|
| Nebulizer gas flow (mL/min) | 1.00 | 0.89 |
| Auxiliary gas flow (L/min) | 1.00 | 1.02 |
| Plasma gas flow (L/min) | 12 | 15.00 |
| RF power (W) | 1400 | 1150 |
| Lens voltage (V) | 9.75 | 6.75 |
| Peristaltic pump rate (rpm) | 1.60 | 2.00 |
| Metal | Landfill Soil—Metal Concentration (mg/100 g) | Control Soil—Metal Concentration (mg/100 g) |
|---|---|---|
| Cr | 75.1 ± 3.7 | 49.6 ± 8.2 |
| Cu | 25.8 ± 1.2 | 15.8 ± 1.1 |
| Mn | 339 ± 3.1 | 291 ± 10 |
| Pb | 168 ± 1.9 | 66.9 ± 0.26 |
| Zn | 294 ± 2.6 | 101 ± 8.4 |
| Metal | BCF | TF | ||
|---|---|---|---|---|
| Plants Grown on Landfill Oil | Control Plants | Plants Grown on Landfill Soil | Control Plants | |
| Cr | 1.41 | 0.49 | 0.25 | 0.33 |
| Cu | 1.67 | 1.53 | 0.80 | 0.65 |
| Mn | 0.64 | 1.25 | 0.43 | 0.69 |
| Pb | 1.03 | 0.87 | 0.32 | 0.48 |
| Zn | 0.47 | 0.33 | 0.42 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masotla, M.K.L.; Melato, F.A.; Mokgalaka-Fleischmann, N.S. Extraction Potential of Lolium perenne L. (Perennial Rye Grass) for Metals in Landfill Soil: Its Tolerance and Defense Strategies. Minerals 2023, 13, 873. https://doi.org/10.3390/min13070873
Masotla MKL, Melato FA, Mokgalaka-Fleischmann NS. Extraction Potential of Lolium perenne L. (Perennial Rye Grass) for Metals in Landfill Soil: Its Tolerance and Defense Strategies. Minerals. 2023; 13(7):873. https://doi.org/10.3390/min13070873
Chicago/Turabian StyleMasotla, Mmatsheko Kgaladi Leah, Funzani Asnath Melato, and Ntebogeng Sharon Mokgalaka-Fleischmann. 2023. "Extraction Potential of Lolium perenne L. (Perennial Rye Grass) for Metals in Landfill Soil: Its Tolerance and Defense Strategies" Minerals 13, no. 7: 873. https://doi.org/10.3390/min13070873