Laboratory Study on the Effectiveness of Limestone and Cementitious Industrial Products for Acid Mine Drainage Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Study Site
2.2. Physical, Chemical, and Mineralogical Material Characterizations
2.3. Toxicity Characteristic Leaching Procedure and Kinetic Column Tests
3. Results and Discussion
3.1. Physical and Chemical Characteristics
3.2. Mineralogical Composition of the Mine Tailings
3.3. Comparison of the Geochemical Behavior of Unamended and Amended Tailings
3.3.1. Results of Toxicity Characteristic Leaching Procedure
3.3.2. Kinetic Column Tests
4. Discussion
- i.
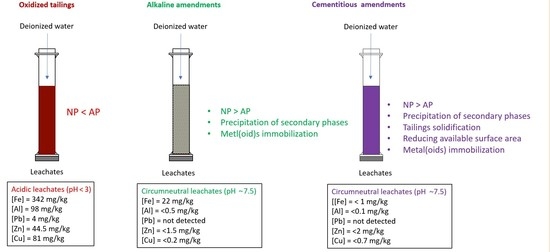
- Alkaline amendments: adding alkaline materials, such as limestone, enhances the neutralization potential of the mixture. This shifts the balance between the acid-generating potential and neutralization potential in a given tailing [20,85]. Acid buffering is reflected by an increase in the alkalinity and pH of the leachates (Figure 4). Changing the geochemical conditions of the system influences the elemental deportment. This means that a number of secondary phases may precipitate [13], the most notable of which are Fe-oxyhydroxides such as ferrihydrite and goethite, and sulfates such as jarosite [68,86]. Although several mechanisms are responsible for metal(loid) attenuation, the most discussed mechanisms are Fe-oxyhydroxide precipitation, and sorption mechanisms [87,88,89]. Moreover, saturation indexes for ferrihydrite and goethite calculated using Visual Minteq (V3.1) showed that these two minerals will likely precipitate (saturation index >0) for the amended tailings (Figure 9A,B). However, for the unamended tailings, these two mineral species will remain in solution (saturation index <0). Dissolution for Fe-oxyhydroxides within the unamended tailings and their precipitation within the amended tailings explains the high concentrations of Fe analyzed within the leachates from the Joutel tailings (Figure 6A).
- ii.
- Cementitious amendments: the same mechanisms discussed within alkaline amendments also occur within cementitious amendments, as they also neutralize acid and increase pH (Figure 4). However, the use of cementitious additives also improves the mechanical properties of the mixtures [23,47,50]. The use of cement and industrial byproducts increases the cohesion and the long-term impermeabilization of the mixture [15]. Consequently, contaminant mobility and diffusion are significantly limited by (i) physical trapping, and (ii) reducing the reactive surface area of the mixture. Tailing solidification reduces the available surface area of sulfide minerals, and thus their reactivity [35,36].

5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bussière, B.; Aubertin, M.; Mbonimpa, M.; Molson, J.W.; Chapuis, R.P. Field experimental cells to evaluate the hydrogeological behaviour of oxygen barriers made of silty materials. Can. Geotech. J. 2007, 44, 245–265. [Google Scholar] [CrossRef]
- Amar, H.; Benzaazoua, M.; Edahbi, M.; Villeneuve, M.; Joly, M.-A.; Elghali, A. Reprocessing feasibility of polymetallic waste rock for cleaner and sustainable mining. J. Geochem. Explor. 2021, 220, 106683. [Google Scholar] [CrossRef]
- Amar, H.; Elghali, A.; Benzaazoua, M. Geochemical behaviour of benign desulphurised waste rocks for mine drainage control and sustainable management. J. Geochem. Explor. 2021, 225, 106767. [Google Scholar] [CrossRef]
- Belem, T.; Benzaazoua, M.; Bussière, B. Mechanical behaviour of cemented paste backfill. In Proceedings of the 53rd Candadian Geotechnical Conference, Montreal, QC, Canada, 15–18 October 2000; pp. 373–380. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G.; Paktunc, D.; Gould, W.D.; Johnson, D.B. The geochemistry of acid mine drainage. In Treatise on Geochemistry; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Amar, H.; Benzaazoua, M.; Elghali, A.; Bussière, B.; Duclos, M. Upstream environmental desulphurisation and valorisation of waste rocks as a sustainable AMD management approach. J. Geochem. Explor. 2020, 215, 106555. [Google Scholar] [CrossRef]
- Evangelou, V.; Zhang, Y. A review: Pyrite oxidation mechanisms and acid mine drainage prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Dmitrijeva, M.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Metcalfe, A.V.; Kamenetsky, M.; Kamenetsky, V.S.; Gilbert, S. Multivariate Statistical Analysis of Trace Elements in Pyrite: Prediction, Bias and Artefacts in Defining Mineral Signatures. Minerals 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Cravotta, C.A., III. Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 1: Constituent quantities and correlations. Appl. Geochem. 2008, 23, 166–202. [Google Scholar] [CrossRef]
- España, J.S.; Pamo, E.L.; Pastor, E.S.; Andrés, J.R.; Rubí, J.A.M. The Removal of Dissolved Metals by Hydroxysulphate Precipitates during Oxidation and Neutralization of Acid Mine Waters, Iberian Pyrite Belt. Aquat. Geochem. 2006, 12, 269–298. [Google Scholar] [CrossRef]
- Jambor, J.; Dutrizac, J.; Groat, L.; Raudsepp, M. Static tests of neutralization potentials of silicate and aluminosilicate minerals. Environ. Geol. 2002, 43, 1–17. [Google Scholar]
- Parbhakar-Fox, A.; Lottermoser, B.G. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Aubertin, M.; McKenna, G. Tailings disposal challenges and prospects for oil sands mining operations. In Geo-Chicago 2016; Geo-Institute of ASCE: Reston, VA, USA, 2016; pp. 359–371. [Google Scholar]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Genty, T. In Situ Effectiveness of Alkaline and Cementitious Amendments to Stabilize Oxidized Acid-Generating Tailings. Minerals 2019, 9, 314. [Google Scholar] [CrossRef] [Green Version]
- Forján, R.; Asensio, V.; Rodríguez-Vila, A.; Covelo, E.F. Effect of amendments made of waste materials in the physical and chemical recovery of mine soil. J. Geochem. Explor. 2014, 147, 91–97. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Laboratory evaluation of the use of alkaline phosphate wastes for the control of acidic mine drainage. Mine Water Environ. 2009, 28, 206. [Google Scholar] [CrossRef]
- Jang, A.; Kim, I.S. Solidification and stabilization of Pb, Zn, Cd and Cu in tailing wastes using cement and fly ash. Miner. Eng. 2000, 13, 1659–1662. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, M.C. Solidification of arsenic and heavy metal containing tailings using cement and blast furnace slag. Environ. Geochem. Health 2011, 33 (Suppl. 1), 151–158. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bartzas, G.; Paspaliaris, I. Efficiency of limestone and red mud barriers: Laboratory column studies. Miner. Eng. 2004, 17, 183–194. [Google Scholar] [CrossRef]
- Mackie, A.L.; Walsh, M.E. Investigation into the use of cement kiln dust in high density sludge (HDS) treatment of acid mine water. Water Res. 2015, 85, 443–450. [Google Scholar] [CrossRef]
- Mylona, E.; Xenidis, A.; Paspaliaris, I. Inhibition of acid generation from sulphidic wastes by the addition of small amounts of limestone. Miner. Eng. 2000, 13, 1161–1175. [Google Scholar] [CrossRef]
- Nehdi, M.; Tariq, A. Stabilization of sulphidic mine tailings for prevention of metal release and acid drainage using cementitious materials: A review. J. Environ. Eng. Sci. 2007, 6, 423–436. [Google Scholar] [CrossRef]
- Rodríguez, L.; Gómez, R.; Sánchez, V.; Villaseñor, J.; Alonso-Azcárate, J. Performance of waste-based amendments to reduce metal release from mine tailings: One-year leaching behaviour. J. Environ. Manag. 2018, 209, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sarkkinen, M.; Kujala, K.; Gehör, S. Efficiency of MgO activated GGBFS and OPC in the stabilization of highly sulfidic mine tailings. J. Sustain. Min. 2019, 18, 115–126. [Google Scholar] [CrossRef]
- Stouraiti, C.; Xenidis, A.; Paspaliaris, I. Reduction of Pb, Zn and Cd Availability from Tailings and Contaminated Soils by the Application of Lignite Fly Ash. Water Air Soil Pollut. 2002, 137, 247–265. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Romano, S.; Vagliasindi, F.G. Stabilisation/Solidification of soils contaminated by mining activities: Influence of barite powder and grout content on γ-radiation shielding, unconfined compressive strength and 232Th immobilisation. J. Geochem. Explor. 2017, 174, 140–147. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Li, X.D.; Poon, C.S.; Sun, H.; Lo, I.M.C.; Kirk, D.W. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef]
- Mitchell, K.; Trakal, L.; Sillerova, H.; Avelar-González, F.J.; Guerrero-Barrera, A.L.; Hough, R.; Beesley, L. Mobility of As, Cr and Cu in a contaminated grassland soil in response to diverse organic amendments; a sequential column leaching experiment. Appl. Geochem. 2018, 88, 95–102. [Google Scholar] [CrossRef]
- Mu, Y.; Saffarzadeh, A.; Shimaoka, T. Utilization of waste natural fishbone for heavy metal stabilization in municipal solid waste incineration fly ash. J. Clean. Prod. 2018, 172, 3111–3118. [Google Scholar] [CrossRef]
- Park, C.-K. Hydration and solidification of hazardous wastes containing heavy metals using modified cementitious materials. Cem. Concr. Res. 2000, 30, 429–435. [Google Scholar] [CrossRef]
- Pesonen, J.; Yliniemi, J.; Illikainen, M.; Kuokkanen, T.; Lassi, U. Stabilization/solidification of fly ash from fluidized bed combustion of recovered fuel and biofuel using alkali activation and cement addition. J. Environ. Chem. Eng. 2016, 4, 1759–1768. [Google Scholar] [CrossRef]
- Simon, L. Stabilization of metals in acidic mine spoil with amendments and red fescue (Festuca rubra L.) growth. Environ. Geochem. Health 2005, 27, 289–300. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Z.; Al-Tabbaa, A. PC-based and MgO-based binders stabilised/solidified heavy metal-contaminated model soil: Strength and heavy metal speciation in early stage. Géotechnique 2018, 68, 1025–1030. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Jin, F.; Al-Tabbaa, A. The performance of blended conventional and novel binders in the in-situ stabilisation/solidification of a contaminated site soil. J. Hazard. Mater. 2015, 285, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Du, Y.-J.; Li, F.-S.; Li, C.-P.; Yan, X.-L.; Arulrajah, A.; Wang, F.; Song, D.-J. In-situ solidification/stabilization of heavy metals contaminated site soil using a dry jet mixing method and new hydroxyapatite based binder. J. Hazard. Mater. 2019, 369, 353–361. [Google Scholar] [CrossRef]
- Zanuzzi, A.; Faz, A.; Acosta, J.A. Chemical stabilization of metals in the environment: A feasible alternative for remediation of mine soils. Environ. Earth Sci. 2013, 70, 2623–2632. [Google Scholar] [CrossRef]
- Belem, T.; Benzaazoua, M. Design and application of underground mine paste backfill technology. Geotech. Geol. Eng. 2008, 26, 147–174. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Belem, T.; Bussière, B. Chemical factors that influence the performance of mine sulphidic paste backfill. Cem. Concr. Res. 2002, 32, 1133–1144. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Demers, I.; Aubertin, M.; Fried, É.; Blier, A. Integrated mine tailings management by combining environmental desulphurization and cemented paste backfill: Application to mine Doyon, Quebec, Canada. Miner. Eng. 2008, 21, 330–340. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Marion, P.; Picquet, I.; Bussière, B. The use of pastefill as a solidification and stabilization process for the control of acid mine drainage. Miner. Eng. 2004, 17, 233–243. [Google Scholar] [CrossRef]
- Peyronnard, O.; Benzaazoua, M. Alternative by-product based binders for cemented mine backfill: Recipes optimisation using Taguchi method. Miner. Eng. 2012, 29, 28–38. [Google Scholar] [CrossRef]
- Duchesne, J.; Reardon, E. Determining controls on element concentrations in cement kiln dust leachate. Waste Manag. 1998, 18, 339–350. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Yi, Y.; Al-Tabbaa, A. Process envelopes for stabilisation/solidification of contaminated soil using lime–slag blend. Environ. Sci. Pollut. Res. 2011, 18, 1286–1296. [Google Scholar] [CrossRef]
- Holmström, H.; Ljungberg, J.; Öhlander, B. Role of carbonates in mitigation of metal release from mining waste. Evidence from humidity cells tests. Environ. Geol. 1999, 37, 267–280. [Google Scholar] [CrossRef]
- Ichrak, H.; Mostafa, B.; Abdelkabir, M.; Bruno, B. Effect of cementitious amendment on the hydrogeological behavior of a surface paste tailings’ disposal. Innov. Infrastruct. Solut. 2016, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Kogbara, R.B.; Al-Tabbaa, A. Mechanical and leaching behaviour of slag-cement and lime-activated slag stabilised/solidified contaminated soil. Sci. Total Environ. 2011, 409, 2325–2335. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Sepúlveda, R.; Esteban, E.; Beesley, L. Efficiency of organic and mineral based amendments to reduce metal[loid]mobility and uptake (Lolium perenne) from a pyrite-waste contaminated soil. J. Geochem. Explor. 2017, 174, 46–52. [Google Scholar] [CrossRef]
- Paradis, M.; Duchesne, J.; Lamontagne, A.; Isabel, D. Using red mud bauxite for the neutralization of acid mine tailings: A column leaching test. Can. Geotech. J. 2006, 43, 1167–1179. [Google Scholar] [CrossRef]
- Srivastava, S.; Chaudhary, R.; Khale, D. Influence of pH, curing time and environmental stress on the immobilization of hazardous waste using activated fly ash. J. Hazard. Mater. 2008, 153, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Rao, F.; Song, S.; Zhang, Y. Immobilization forms of ZnO in the solidification/stabilization (S/S) of a zinc mine tailing through geopolymerization. J. Mater. Res. Technol. 2019. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Marschner, B.; Itanna, F.; Gebrekidan, H. Effects of biochar and alkaline amendments on cadmium immobilization, selected nutrient and cadmium concentrations of lettuce (Lactuca sativa) in two contrasting soils. SpringerPlus 2016, 5, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.; Wen, J.; Zeng, G.; Zhang, T.; Huang, F.; Qin, H.; Tian, S. A comparative study for the stabilisation of heavy metal contaminated sediment by limestone, MnO2 and natural zeolite. Environ. Sci. Pollut. Res. 2017, 24, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Blowes, D.W.; Jambor, J.L.; Hanton-Fong, C.J.; Lortie, L.; Gould, W.D. Geochemical, mineralogical and microbiological characterization of a sulphide-bearing carbonate-rich gold-mine tailings impoundment, Joutel, Québec. Appl. Geochem. 1998, 13, 687–705. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Genty, T. Spatial Mapping of Acidity and Geochemical Properties of Oxidized Tailings within the Former Eagle/Telbel Mine Site. Minerals 2019, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Miller, P.; Zuiderwyk, M.; Reid, A. SEM Image Analysis in the Determination of Modal Assays, Mineral Associations and Mineral Liberation. 1983. Available online: http://pdf.library.laurentian.ca/medb/conf/Sudbury99/AcidicDrainage/AD8.PDF (accessed on 15 March 2021).
- Halim, C.E.; Amal, R.; Beydoun, D.; Scott, J.A.; Low, G. Evaluating the applicability of a modified toxicity characteristic leaching procedure (TCLP) for the classification of cementitious wastes containing lead and cadmium. J. Hazard. Mater. 2003, 103, 125–140. [Google Scholar] [CrossRef]
- US EPA. EPA Method 1311; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Bussière, B.; Benzaazoua, M.; Aubertin, M.; Mbonimpa, M. A laboratory study of covers made of low-sulphide tailings to prevent acid mine drainage. Environ. Geol. 2004, 45, 609–622. [Google Scholar] [CrossRef]
- Demers, I.; Bussière, B.; Aachib, M.; Aubertin, M. Repeatability evaluation of instrumented column tests in cover efficiency evaluation for the prevention of acid mine drainage. Water Air Soil Pollut. 2011, 219, 113–128. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Kennedy, C.; Parwani, R.; Graham, S. The role of hardpan formation on the reactivity of sulfidic mine tailings: A case study at Joutel mine (Québec). Sci. Total Environ. 2019, 654, 118–128. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ 3.0 User Guide; KTH, Department of Land and Water Recources: Stockholm, Sweden, 2011. [Google Scholar]
- Adam, K.; Kourtis, A.; Gazea, B.; Kontopoulos, A. Evaluation of static tests used to predict the potential for acid drainage generation at sulphide mine sites. Min. Technol. IMM Trans. Sect. A 1997, 106, A1–A8. [Google Scholar]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Brough, C.; Strongman, J.; Bowell, R.; Warrender, R.; Prestia, A.; Barnes, A.; Fletcher, J. Automated environmental mineralogy; the use of liberation analysis in humidity cell testwork. Miner. Eng. 2017, 107, 112–122. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Bussière, B.; Villarraga-Gómez, H. Determination of the available acid-generating potential of waste rock, part I: Mineralogical approach. Appl. Geochem. 2018, 99, 31–41. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Bouzahzah, H. Determination of the available acid-generating potential of waste rock, part II: Waste management involvement. Appl. Geochem. 2019, 100, 316–325. [Google Scholar] [CrossRef]
- Erguler, Z.A.; Kalyoncu Erguler, G. The effect of particle size on acid mine drainage generation: Kinetic column tests. Miner. Eng. 2015, 76, 154–167. [Google Scholar] [CrossRef]
- Paktunc, A.; Davé, N. Mineralogy of pyritic waste rock leached by column experiments and prediction of acid mine drainage. In Applied Mineralogy; Rammlmair, D., Ed.; Balkema: Rotterdam, The Netherlands, 2000; pp. 621–623. [Google Scholar]
- Benzaazoua, M.; Bussière, B.; Dagenais, A.-M.; Archambault, M. Kinetic tests comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ. Geol. 2004, 46, 1086–1101. [Google Scholar] [CrossRef]
- Cravotta, C.A., III. Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 2: Geochemical controls on constituent concentrations. Appl. Geochem. 2008, 23, 203–226. [Google Scholar] [CrossRef]
- Godbout, J.; Bussière, B.; Belem, T. Evolution of Cemented Paste Backfill Saturated Hydraulic Conductivity at Early Curing Time. 2007. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.484.1839&rep=rep1&type=pdf (accessed on 15 March 2021).
- Lapakko, K.A.; Engstrom, J.N.; Antonson, D.A. Effects of particle size on drainage quality from three lithologies. In Proceedings of the 7th International Conference on Acid Rock Drainage (ICARD), St. Louis, MO, USA, 26–30 March 2006; pp. 1026–1050. [Google Scholar]
- Cravotta, C., III. Secondary iron-sulfate minerals as sources of sulfate and acidity: Geochemical evolution of acidic ground water at a reclaimed surface coal mine in Pennsylvania. In Environmental Geochemistry of Sulfide Oxidation; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
- Fox, L.E. The solubility of colloidal ferric hydroxide and its relevance to iron concentrations in river water. Geochim. Cosmochim. Acta 1988, 52, 771–777. [Google Scholar] [CrossRef]
- Québec, G.d. Directive 019 sur L’Industrie Minière; Ministère Du Développement Durableenvironement Et Parcsgouvernement Du Québec: Quebec City, QC, Canada, 2012; p. 105. [Google Scholar]
- Doye, I.; Duchesne, J. Neutralisation of acid mine drainage with alkaline industrial residues: Laboratory investigation using batch-leaching tests. Appl. Geochem. 2003, 18, 1197–1213. [Google Scholar] [CrossRef]
- Kalia, N.; Balakotaiah, V. Effect of medium heterogeneities on reactive dissolution of carbonates. Chem. Eng. Sci. 2009, 64, 376–390. [Google Scholar] [CrossRef]
- Li, M.G.; Bernier, L. Contributions of Carbonates and Silicates to Neutralization Observed in Laboratory Tests and Their Field Implications; Belzile, N., Yearwood, P., Goldsack, D., Eds.; CIM: Montreal, QC, Canada, 1999; pp. 59–68. [Google Scholar]
- Fatahi, B.; Khabbaz, H. Influence of Chemical Stabilisation on Permeability of Municipal Solid Wastes. Geotech. Geol. Eng. 2015, 33, 455–466. [Google Scholar] [CrossRef]
- De Andrade, R.P.; Figueiredo, B.R.; de Mello, J.W.V.; Santos, J.C.Z.; Zandonadi, L.U. Control of geochemical mobility of arsenic by liming in materials subjected to acid mine drainage. J. Soils Sediments 2008, 8, 123–129. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation. Rev. Mineral. 1997, 35, 361–390. [Google Scholar]
- Manceau, A. The mechanism of anion adsorption on iron oxides: Evidence for the bonding of arsenate tetrahedra on free Fe(O, OH)6 edges. Geochim. Cosmochim. Acta 1995, 59, 3647–3653. [Google Scholar] [CrossRef]
- Manceau, A.; Charlet, L.; Boisset, M.C.; Didier, B.; Spadini, L. Sorption and speciation of heavy metals on hydrous Fe and Mn oxides. From microscopic to macroscopic. Appl. Clay Sci. 1992, 7, 201–223. [Google Scholar] [CrossRef]
- Manceau, A.; Combes, J.M. Structure of Mn and Fe oxides and oxyhydroxides: A topological approach by EXAFS. Phys. Chem. Miner. 1988, 15, 283–295. [Google Scholar] [CrossRef]
- Ghosh, A.; Mukiibi, M.; Ela, W. TCLP underestimates leaching of arsenic from solid residuals under landfill conditions. Environ. Sci. Technol. 2004, 38, 4677–4682. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Mateus, R.; Camões, A.F.; Bragança, L.; Branco, F.G. Comparative environmental life-cycle analysis of concretes using biomass and coal fly ashes as partial cement replacement material. J. Clean. Prod. 2016, 112, 2221–2230. [Google Scholar] [CrossRef] [Green Version]








| Problematic | Amendments | Country | Reference |
|---|---|---|---|
| Acid-generating tailings | 5% and 10% limestone, 5% OPC, 5% (1/2 OPC-1/2 FA) | Canada | [15] |
| Contaminated mine soils | Sludges from a purification plant and a bleach plant | Spain | [16] |
| Acid-generating mine tailings | Alkaline phosphate wastes | Morocco | [17] |
| Contaminated mine tailings | Ordinary Portland cement and furnace slag | Korea | [18,19] |
| Synthetic-acid-generating tailings | Limestone and red mud | Greece | [20] |
| Acid mine drainage | Cement kiln dust | Canada | [21] |
| Sulfidic mine waste | Limestone | Canada | [22] |
| Acid-generating mine wastes | Cementitious materials | Review paper | [23] |
| Contaminated mine tailings | Sugar foam, lime-rich waste, drinking-water-treatment sludge, olive mill waste and paper mill sludge | Spain | [24] |
| Highly sulfidic mine tailings | MgO-activated ground granulated blast-furnace slag and ordinary Portland cement | Finland | [25] |
| Tailings and contaminated soils | Fly ash | Greece | [26] |
| Radioactive-contaminated soils | Portland cement and barite powder | Italy | [27] |
| Contaminated materials | Organic matter, alkaline material | Review paper | [28] |
| Waste materials | Ordinary Portland cement and pulverized fly ash | Not indicated | [29] |
| Wood-ash-contaminated grassland soil | Biochar | Not indicated | [30] |
| Municipal solid-waste incineration | Waste fishbone | Japan | [31] |
| Synthetic-contaminated materials | Ordinary Portland cement, clinker kiln dust modified OPC, cement kiln dust | South Korea | [32] |
| Fuel–biofuel fly ash | Ordinary Portland cement and alkali activation | Finland | [33] |
| Acidic and phytotoxic mine spoil | Lime and nitrogen (NH4NO3) | Hungary | [34] |
| Contaminated soils | Portland cement, MgO-based cement, ground granulated blast-furnace slag | United Kingdom | [35] |
| Contaminated soils | Portland cement, ground granulated blast-furnace slag, pulverized fuel ash, MgO, and zeolite | United Kingdom | [36] |
| Lead–zinc smelting slag | Geopolymerization | China | [37] |
| Contaminated soils | Superphosphate and calcium oxide | China | [38] |
| Mine soils | Lime, organic amendments | Spain | [39] |
| Mine backfill | Ordinary Portland cement, cement kiln dust, ground granulated blast furnace slag, waste glass, copper slag, wood bottom ash and coal fly ash | Canada | [44] |
| Synthetic-contaminated soils | Lime-slag | United Kingdom | [47] |
| Acid generating mine tailings | |||
| Carbonates | Sweden | [48] | |
| Acid-generating mine tailings | 2% Portland cement | Canada | [49] |
| Synthetic-contaminated soils | Ground granulated blast-furnace slag and hydrated lime | United Kingdom | [50] |
| Pyrite-waste-contaminated soil | Iron- and phosphorus-based amendments | Spain | [51] |
| Acid-generating mine tailings | Red mud bauxite | Canada | [52] |
| Contaminated sludge generated from common effluent treatment plant | Ordinary Portland cement, fly ash and sludge | India | [53] |
| Zinc mine tailings | Geopolymerization | China | [54] |
| Cadmium-spiked soils | Biochar and alkaline amendments | Ethiopia | [55] |
| Contaminated sediments | Limestone, MnO2, and natural zeolite | China | [56] |
| Column | Amendment Type | Formulation |
|---|---|---|
| C1 | Reference | Mine tailings |
| C2 | Alkaline amendment | 20 wt.% LS |
| C3 | Alkaline amendment | 10 wt.% LS |
| C4 | Cementitious amendment | 5 wt.% (50%FAF-50%CVK) |
| C5 | Cementitious amendment | 5 wt.% (20%OPC-40%CVK-40%FAF) |
| C6 | Cementitious amendment | 5 wt.% (80%OPC-20%CVK) |
| C7 | Cementitious amendment | 5 wt.% (20%OPC-40%CVK-40%FA) |
| Units | DL (ppm) | MT | LS | OPC | FA | CVK | FAF | ||
|---|---|---|---|---|---|---|---|---|---|
| Chemical composition | Al | % | 60 | 1.73 | 0.30 | 2.75 | 4.69 | 3.84 | 11.91 |
| Ca | 60 | 3.00 | 33.82 | 49.07 | 7.58 | 34.88 | 2.87 | ||
| Mg | 15 | 0.27 | 2.35 | 1.18 | 1.05 | 1.56 | 0.58 | ||
| Mn | 5 | 0.231 | 0.03 | 0.06 | 0.42 | 0.12 | 0.02 | ||
| Na | 1 | 0.972 | 0.125 | 0.16 | 1.96 | 0.45 | 0.45 | ||
| K | 1 | 0.267 | 0.245 | 0.43 | 1.85 | 0.32 | 1.38 | ||
| Fe | 10 | 19.30 | 0.483 | 2.23 | 2.24 | 3.31 | 13.43 | ||
| Li | 5 | ≤DL | ≤DL | ≤DL | 0.002 | ≤DL | ≤DL | ||
| Pb | 5 | 0.042 | ≤DL | ≤DL | ≤DL | 0.05 | ≤DL | ||
| As | 5 | ≤DL | ≤DL | 0.005 | ≤DL | 0.006 | ≤DL | ||
| Cr | 5 | 0.01 | 0.004 | 0.007 | 0.006 | 0.014 | 0.02 | ||
| Cu | 10 | 0.001 | ≤DL | 0.007 | ≤DL | 0.031 | ≤DL | ||
| Zn | 55 | 0.007 | ≤DL | 0.049 | 0.070 | 0.15 | 0.19 | ||
| S (total) | 200 | 4.36 | NA | 1.737 | 0.431 | 3.33 | 0.38 | ||
| S (sulfates) | 4.20 | NA | NA | NA | 3.33 | 0.38 | |||
| C | 900 | 0.2 | NA | NA | NA | 1.29 | 2.69 | ||
| Physical characteristics | D10 | µm | 1.8 | 50 | 4.2 | 82 | 5.78 | 6.07 | |
| D30 | 4.7 | 250 | 11.3 | 180 | 14.3 | 12.8 | |||
| D90 | 30.4 | 4500 | 46.7 | 1500 | 104.17 | 89.12 |
| pH | EC (mS/cm) | Al | Fe | Ni | S | Zn | |
|---|---|---|---|---|---|---|---|
| C1 | 2.50 | 7.81 | 0.40 | 0.50 | 0.01 | 766 | 0.60 |
| C2 | 6.46 | 9.37 | 0.01 | 0.06 | <DL | 567 | 0.34 |
| C3 | 5.58 | 8.84 | 0.01 | 0.05 | < DL | 608 | 0.33 |
| C4 | 5.00 | 8.15 | 0.04 | 0.05 | < DL | 664 | 0.30 |
| C5 | 5.05 | 8.25 | 0.02 | 0.05 | < DL | 669 | 0.60 |
| C6 | 4.92 | 8.17 | 0.03 | 0.08 | < DL | 696 | 0.31 |
| C7 | 4.93 | 8.05 | 0.05 | 0.04 | < DL | 695 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Bussière, B. Laboratory Study on the Effectiveness of Limestone and Cementitious Industrial Products for Acid Mine Drainage Remediation. Minerals 2021, 11, 413. https://doi.org/10.3390/min11040413
Elghali A, Benzaazoua M, Bouzahzah H, Bussière B. Laboratory Study on the Effectiveness of Limestone and Cementitious Industrial Products for Acid Mine Drainage Remediation. Minerals. 2021; 11(4):413. https://doi.org/10.3390/min11040413
Chicago/Turabian StyleElghali, Abdellatif, Mostafa Benzaazoua, Hassan Bouzahzah, and Bruno Bussière. 2021. "Laboratory Study on the Effectiveness of Limestone and Cementitious Industrial Products for Acid Mine Drainage Remediation" Minerals 11, no. 4: 413. https://doi.org/10.3390/min11040413