Leaching of Rare Earth Elements from NdFeB Magnets without Mechanical Pretreatment by Sulfuric (H2SO4) and Hydrochloric (HCl) Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
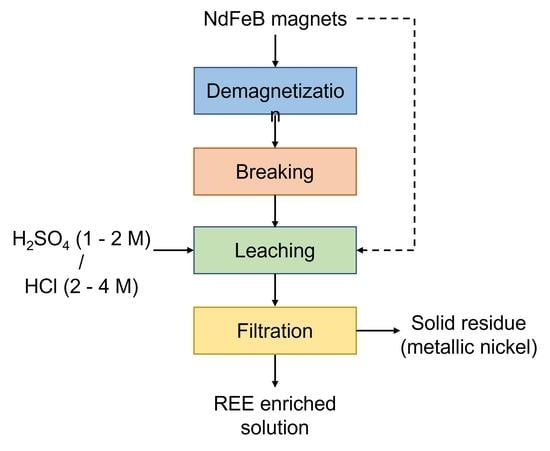
2.2. Leaching Procedure
2.2.1. Thermodynamic Analysis of Nickel Digestion in Acids
2.2.2. Analysis of Liquors
2.2.3. Analysis of Acid Concentrations
2.2.4. Analysis of Solid Residue
3. Results and Discussion
3.1. Theoretical Studies on Selective Leaching
3.2. Preliminary Leaching Tests and Assumptions of the Leaching Process
- –
- the crushing and milling of magnets, as well as oxidative roasting unit operations will be omitted;
- –
- – broken demagnetized magnets or whole magnets without demagnetization will be used as feed material in the leaching process;
- –
- the leaching process will lead to the complete dissolution of the magnets, and it will leave nickel in the solid phase;
- –
- due to the physical form of the feed material, mechanical stirring is impossible; therefore, a rotary reactor will be used,
- –
- the reaction surface (the surface of magnets not covered by nickel layer) is unknown and can change randomly;
- –
- feed material is not homogenous and, therefore, only concentration of metals, without efficiency, will be determined in the leaching experiments;
- –
- the concentration of leaching agents (hydrochloric and sulfuric acid) will be high enough to dissolve magnets, but not too high in order to avoid a violent evolution of hydrogen;
- –
- the solid-to-liquid ratio will be defined by the requirement that a slight concentration of acid after the completed leaching process should be maintained;
- –
- a moderate leaching temperature (≤60 °C) will be applied.
3.3. Leaching in H2SO4 Solutions
3.4. Leaching in HCl Solutions
4. Final Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaya, E.E.; Kaya, O.; Stopic, S.; Gürmen, S.; Friedrich, B. NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets. Metals 2021, 11, 716. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions. Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability. Brussels. 3 September 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0474&from=EN (accessed on 21 October 2021).
- Charles, R.G.; Douglas, P.; Dowling, M.; Liversage, G.; Davies, M.L. Towards Increased Recovery of Critical Raw Materials from WEEE—Evaluation of CRMs at a component level and pre-processing methods for interface optimisation with recovery processes. Resour. Conserv. Recycl. 2020, 161, 104923. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brűck, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Ni’am, A.C.; Wang, Y.-F.; Chen, S.-W.; You, S.-J. Recovery of rare earth elements from waste permanent magnet (WPMs) via selective leaching using the Taguchi method. J. Taiwan Inst. Chem. Eng. 2019, 91, 137–145. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Gu, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Hiroshige, Y.; Nemoto, T. Rare-earth Magnet Recycling. Hitachi Rev. 2013, 62, 452–455. [Google Scholar]
- Goonan, T.G. Rare Earth Elements-End Use and Recyclability; Scientific Investigations Report; Scientific US Geological Survey: Reston, VA, USA, 2009. Available online: https://pubs.usgs.gov/sir/2011/5094/pdf/sir2011-5094.pdf (accessed on 1 December 2021).
- Chancerel, P.; Rotter, S. Recycling-oriented characterization of small waste electrical and electronic equipment. Waste Manag. 2009, 29, 2336–2352. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Anderson, C.D.; Anderson, C.G.; Taylor, P.R. Survey of recycled rare earths metallurgical processing. Can. Metall. Q. 2013, 52, 249–256. [Google Scholar] [CrossRef]
- Ferron, C.J.; Henry, P. A review of the recycling of rare earth metals. Can. Metall. Q. 2015, 54, 388–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical Recovery of Rare Earth Elements from NdFeB Permanent Magnet Scrap: A Review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Lyman, J.W.; Palmer, G.R. Recycling of Neodymium Iron Boron Magnet Scrap; Report of Investigation RI 9481; US Bureau of Mines: Washington, DC, USA, 1993. [Google Scholar]
- Ellis, T.W.; Schmidt, F.A.; Jones, L.L. Methods and opportunities in the recycling of rare earth based materials. In Symposium on Metals and Materials Waste Reduction: Recovery and Remediation; Liddell, K.C., Bautista, R.G., Orth, R.J., Eds.; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 1994; pp. 199–206. [Google Scholar]
- Chung, K.W.; Kim, C.J.; Yoon, H.S. Novel Extraction process of rare earth elements from NdFeB powders via alkaline treatment. Arch. Metall. Mater. 2015, 60, 1301–1305. [Google Scholar] [CrossRef]
- Abrahami, S.T.; Xiao, Y.; Yang, Y. Rare-earth elements recovery from post-consumer hard-disc drives. Miner. Process. Extr. Metall. 2015, 124, 106–115. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Field, K.D.; Emmert, M.H. Rare earth recovery from end-of-life motors employing green chemistry design principles. Green. Chem. 2016, 18, 753–759. [Google Scholar] [CrossRef]
- Nakashima, K.; Kubota, F.; Maruyama, T.; Goto, M. Ionic liquids as a novel solvent for lanthanide extraction. Anal. Sci. 2003, 19, 1097–1098. [Google Scholar] [CrossRef] [Green Version]
- Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Recent advances in extraction and separation of rare-earth metals using ionic liquids. J. Chem. Eng. Jpn. 2011, 44, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Kubota, F.; Baba, Y.; Goto, M. Application of ionic liquids for the separation of rare earth metals. Solvent Extr. Res. Dev. Jpn. 2012, 19, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of ionic liquids in hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343. [Google Scholar] [CrossRef] [Green Version]
- Dupont, D.; Binnemans, K. Recycling of rare earths from NdFeB magnets using combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]. Green Chem. 2015, 17, 2150. [Google Scholar] [CrossRef] [Green Version]
- Makanyire, T.; Sanchez-Segado, S.; Jha, A. Separation and recovery of critical metal ions using ionic liquids. Adv. Manuf. 2016, 4, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Makarova, I.; Soboleva, E.; Osipenko, M.; Kurilo, I.; Laatikainen, M.; Repo, E. Electrochemical leaching of rare-earth elements from spent NdFeB magnets. Hydrometallurgy 2020, 192, 105264. [Google Scholar] [CrossRef]
- Itoh, M.; Masuda, M.; Suzuki, S.; Machida, K.I. Recycling of rare earth sintered magnets as isotropic bonded magnets by melt-spinning. J. Alloys Compd. 2004, 374, 393–396. [Google Scholar] [CrossRef]
- Okabe, T.H.; Takeda, O.; Fukuda, K.; Umetsu, Y. Direct extraction and recovery of neodymium from a magnet scrap. Mater. Trans. 2003, 44, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Phase Equlibrium of the System Ag-Fe-Nd and Nd Extraction from Magnets Scraps using Molten Silver. J. Alloys Compd. 2004, 397, 305–313. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Recovery of neodymium from mixture of magnet scrap and other scrap. J. Alloys Compd. 2006, 408, 387–390. [Google Scholar] [CrossRef]
- Uda, T. Recovery of rare earths from magnet sludge by FeCl2. Mater. Trans. 2002, 43, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Miura, K.; Machida, K.I. Novel rare earth recovery process on Nd-FeB magnet scrap by selective chlorination using NH4Cl. J. Alloys Compd. 2009, 477, 484–487. [Google Scholar] [CrossRef]
- Kumari, A.; Raj, R.; Randhawa, N.S.; Sahu, S.K. Energy efficient process for recovery of rare earths from spent NdFeB magnet by chlorination roasting and water leaching. Hydrometallurgy 2021, 201, 105581. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB magnets using sulfation. selective roasting. and water leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, C.J.; Lee, J.Y.; Kim, S.D.; Lee, J.C. Separation of neodymium from NdFeB permanent magnet scrap. J. Korean Inst. Resour. Recycl. 2003, 12, 57–63. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Pramanik, S.; Sahu, S.K. Recovery of rare earths from spent NdFeB magnets of wind turbine: Leaching and kinetic aspects. Waste Manag. 2018, 75, 486–498. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Wang, J.L.; Wang, H.; Peng, C.; Wilson, B.P.; Lundström, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets. Miner. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- Zakotnik, M.; Harris, I.R.; Williams, A.J. Possible methods of recycling Nd-Fe-B-type magnets using the HD/degassing process. J. Alloys Compd. 2008, 450, 525–531. [Google Scholar] [CrossRef]
- Zakotnik, M.; Harris, I.R.; Williams, A.J. Multiple recycling of Nd-Fe-B-type sintered magnets. J. Alloys Compd. 2009, 469, 314–321. [Google Scholar] [CrossRef]
- Zakotnik, M.; Tudor, C.O. Commercial-scale recycling of NdFeB-type magnets with grain boundary modification yields products with ‘designer properties’ that exceed those of starting materials. Waste Manag. 2015, 44, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wills, B.A. Chapter 7—Grinding Mills. In Wills’ Mineral Processing Technology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 147–179. [Google Scholar]
- Macko, M. Size Reduction by Grinding as an Important Stage in Recycling. In Post-Consumer Waste Recycling and Optimal Production; Damanhuri, E., Ed.; IntechOpen: London, UK, 2012; pp. 274–292. [Google Scholar] [CrossRef]
- Dańczak, A.; Chojnacka, I.; Matuska, S.; Marcola, K.; Leśniewicz, A.; Wełna, M.; Żak, A.; Adamski, Z.; Rycerz, L. The recycling-oriented material characterization of hard disk drives with special emphasis on NdFeB magnets. Physicochem. Probl. Miner. Process. 2018, 54, 363–376. [Google Scholar]
- Lim, K.H.; Choi, C.U.; Moon, G.; Lee, T.H.; Kang, J. Selective Chlorination of Rare Earth Elements from a Nd-Fe-B Magnet Using Zinc Chloride. J. Sustain. Metall. 2022, 7, 794–805. [Google Scholar] [CrossRef]
- Leonowicz, M.; Wysłocki, J.J. Współczesne Magnesy; Wydawnictwo Naukowo Techniczne: Warszawa, Poland, 2005. [Google Scholar]
- Paszowski, L. Wpływ Proszkowej fazy Nd-Fe-B w Formowanych Wtryskowo Kompozytach Magnetycznych o Osnowie Polimerowej na ich Właściwości Magnetyczne i Mechaniczne. Ph.D. Thesis, Politechnika Warszawska, Warszawa, Poland, 2009. [Google Scholar]
- Chmielewski, T.; Czaja, J.; Klinghoffer, O. Potencjometryczne Oznaczanie Kwasu Siarkowego w Obecności Cu(II), Fe(II) i Fe(III); University of Technology: Wrocław, Poland, 1975. [Google Scholar]
- Błok, N. Jakościowa Analiza Chemiczna; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1955; pp. 244–279. [Google Scholar]
- Brzyska, W. Lantanowce i Aktynowc; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1987; p. 16. [Google Scholar]
- Lee, C.H.; Chen, Y.J.; Liao, C.H.; Popuri, S.R.; Tsai, S.L.; Hung, C.E. Selective Leaching Process for Neodymium Recovery from Scrap Nd-Fe-B Magnet. Metall. Mater. Trans. A 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Prakash, V.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Electrochemical Recovery of Rare Earth Elements from Magnets Scraps—A Theoretical Analysis. In Proceedings of the ERES 2014: 1st European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014. [Google Scholar]
- Schults, L.; El-Aziz, A.M.; Barkleit, G.; Mummert, K. Corrosion behavior of NdFeB permanent magnetic alloys. Mater. Sci. Eng. A 1999, 267, 307–313. [Google Scholar] [CrossRef]
- Mao, S.; Yang, H.; Song, Z.; Li, J.; Ying, H.; Sun, K. Corrosion behavior of sintered NdFeB deposited with an aluminum coating. Corros. Sci. 2011, 53, 1887–1894. [Google Scholar] [CrossRef]
- Szymura, S.; Bala, H.; Pawlowska, G.; Rabinovich, Y.; Sergeev, V.; Pokrovskii, D.V. Modification of the magnetic properties and corrosion resistance of Nd-Fe-B permanent magnets with addition of cobalt. J. Less Common Met. 1991, 175, 185–198. [Google Scholar] [CrossRef]







| Method | Description | References |
|---|---|---|
| Hydrometallurgy | Leaching of the magnets or magnets scrap (acidic or alkaline treatment), followed by separation of REEs using solvent extraction, ion exchange technology or selective precipitation | [5,6,15,16,17,18,19] |
| Ionometallurgy | Use of ionic liquids in the liquid–liquid extraction of REEs present in NdFeB magnets | [20,21,22,23,24,25] |
| Electrochemical leaching | Electrochemical leaching of REEs is achieved at lower cell voltages by adding oxalic acid in the sulfuric acid leach solution. Separation of REEs is achieved by precipitation using H2C2O4 | [26] |
| Acid baking with water leaching and ultrasonic spray pyrolysis | Magnets are crushed, grinded and sieved. Nitric acid is used for acid baking without dilution. After calcination, water leaching is used for selective separation of REEs, which is followed by ultrasonic spray pyrolysis | [1] |
| Electroslag remelting | Melting of a relatively large batch of magnetic slag material as a consumable anode or by addition to a molten bath | [16] |
| Melt spinning | Remelting of magnet scraps by induction heating and conversion of the material into a master alloy | [27] |
| Treatment with liquid metals | Selective extraction of neodymium from magnet scrap with liquid magnesium or molten silver | [28,29,30] |
| Chlorination roasting | REE extraction from neodymium magnet sludge by chlorination with FeCl2 | [31,32,33] |
| Sulfation roasting | REE extraction by suitable selective roasting and water leaching treatment after completely transforming powdered samples into sulfate mixture | [34] |
| Oxidation roasting | Oxidation of NdFeB magnets at a high temperature followed by selective leaching. Before roasting, the magnets are demagnetized, crushed and grinded | [35,36,37] |
| Hydrogen decrepitation (HD) process and re-sintering | NdFeB magnet scrap recycling by processing it in hydrogen, then milling, aligning, pressing and re-sintering it | [38,39,40] |
| Leaching Agent (Solution Volume) | Temperature /°C | Acid Concentration /mol·L−1 | Mass of Magnets /g | Form of Magnets |
|---|---|---|---|---|
| H2SO4 (200 mL) | RT, 40, 60 | 1.0 | 13.5 ± 0.3 | Demagnetized, broken |
| H2SO4 (100 mL) | RT, 40, 60 | 2.0 | 13.5 ± 0.3 | Demagnetized, broken |
| H2SO4 (200 mL) | RT, 40, 60 | 1.0 | 40.9 ± 1.5 g Magnet and plate | Whole, without demagnetization |
| H2SO4 (100 mL) | RT, 40, 60 | 2.0 | 40.9 ± 1.5 g Magnet and plate | Whole, without demagnetization |
| HCl (200 mL) | RT, 40 | 2.0 | 13.5 ± 0.3 | Demagnetized, broken |
| HCl (100 mL) | RT, 40 | 4.0 | 13.5 ± 0.3 | Demagnetized, broken |
| HCl (200 mL) | RT, 40 | 2.0 | 40.9 ± 1.5 g Magnet and plate | Whole, without demagnetization |
| HCl (100 mL) | RT, 40 | 4.0 | 40.9 ± 1.5 g Magnet and plate | Whole, without demagnetization |
| Component | Standard Reduction Potential/V |
|---|---|
| Nickel (Ni2+/Ni) | −0.23 |
| Iron (Fe2+/Fe) | −0.44 |
| Boron (H3BO3/B) | −0.89 |
| REEs (Ln3+/Ln) | from −2.52 to −2.25 |
| T/°C | ΔH0/kJ·mol−1 of Me | ΔS0/kJ·K−1·mol−1 of Me | ΔG0/kJ·mol−1 of Me |
|---|---|---|---|
| Ni + 2HCl(a) = NiCl2 + H2(g) | |||
| 20 | −53.8 | −22.5 | −46.0 |
| 40 | −54.7 | −29.5 | −45.4 |
| 60 | −55.6 | −32.5 | −44.8 |
| Ni + H2SO4(a) = NiSO4 + H2(g) | |||
| 20 | −50.6 | 27.0 | −58.5 |
| 40 | −45.2 | 44.9 | −59.3 |
| 60 | −39.4 | 62.9 | −60.3 |
| 1.5 Ni + 4HNO3(a) = 1.5 Ni(NO3)2 + NO(g) + 2 H2O | |||
| 20 | −282.2 | −106.6 | −250.9 |
| 40 | −284.3 | −113.6 | −248.7 |
| 60 | −286.4 | −120.1 | −246.4 |
| Nd + 3HCl(a) = NdCl3(a) + 1.5H2(g) | |||
| 20 | −798.4 | 140.6 | −839.7 |
| 40 | −815.1 | 85.6 | −841.9 |
| 60 | −831.4 | 35.1 | −843.1 |
| Nd + 1.5H2SO4(a) = 0.5Nd2(SO4)3(a) +1.5H2(g) | |||
| 20 | −694.2 | −80.8 | −670.5 |
| 40 | −696.7 | −89.3 | −668.7 |
| 60 | −697.3 | −91.1 | −666.9 |
| Leaching Agent | Temperature | The State of Magnets | B | Co | Fe | Ni | Nd | Pr | Dy | Tb | Total REE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g·L−1 | |||||||||||
| H2SO4 1M | room temp. | demagnetized and broken | 0.535 | 1.70 | 31.6 | 0.0027 | 15.1 | 0.535 | 0.585 | 0.243 | 16.463 |
| whole | 0.237 | 0.769 | 17.5 | 0.0119 | 8.15 | 0.639 | 0.625 | 0.0125 | 9.426 | ||
| 40 °C | demagnetized and broken | 0.605 | 0.740 | 43.4 | 0.0031 | 15.9 | 6.20 | 0.0261 | 0.218 | 22.344 | |
| whole | 0.133 | 0.0047 | 23.7 | 0.0096 | 10.3 | 0.578 | 0.565 | 0.0032 | 11.446 | ||
| 60 °C | demagnetized and broken | 0.656 | 2.22 | 43.3 | 0.0210 | 20.4 | 2.15 | 1.109 | 0.200 | 23.859 | |
| whole | 0.259 | 0.849 | 17.0 | 0.0166 | 8.00 | 0.573 | 0.660 | 0.0209 | 9.2539 | ||
| H2SO4 2M | room temp. | demagnetized and broken | 1.12 | 0.0124 | 86.1 | 0.0445 | 24.0 | 24.2 | 0.159 | 1.12 | 49.479 |
| whole | 0.252 | 0.0056 | 18.1 | 0.0068 | 7.70 | 0.317 | 0.272 | 0.0029 | 8.292 | ||
| 40 °C | demagnetized and broken | 0.950 | 0.0173 | 64.7 | 0.0153 | 18.6 | 20.9 | 0.416 | 0.166 | 40.082 | |
| whole | 0.312 | 0.142 | 20.7 | 0.0105 | 6.76 | 2.52 | 0.567 | 0.0061 | 9.853 | ||
| 60 °C | demagnetized and broken | 1.32 | 4.48 | 74.3 | 0.0169 | 33.1 | 2.77 | 2.72 | - | 38.590 | |
| whole | 0.324 | 0.102 | 21.6 | 0.0123 | 7.24 | 2.45 | 0.643 | 0.0053 | 10.338 | ||
| Leaching Agent | Temperature | The State of Magnets | B | Co | Fe | Ni | Nd | Pr | Dy | Tb | Total REE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g·L−1 | |||||||||||
| HCl 2M | room temp. | demagnetized and broken | 0.570 | 1.09 | 37.9 | 0.0096 | 16.7 | 4.3 | 0.208 | 0.346 | 21.554 |
| whole | 0.218 | 0.124 | 13.45 | 0 | 4.935 | 0.99 | 0.54 | 0.0009 | 6.466 | ||
| 40 °C | demagnetized and broken | 0.620 | 0.66 | 44.2 | 0.0128 | 18.7 | 4.787 | 0.82 | 0.0816 | 24.3886 | |
| whole | 0.281 | 0.975 | 16.55 | 0.0139 | 8.3 | 0.2965 | 0.865 | 0.0063 | 9.4678 | ||
| HCl 4M | room temp. | demagnetized and broken | 0.987 | 0.607 | 38.60 | 0.018 | 24.67 | 1.198 | 1.553 | 0.017 | 27.438 |
| whole | 0.329 | 1.175 | 20.85 | 0.0068 | 8.4 | 0.3385 | 0.935 | 0.001 | 9.674 | ||
| 40 °C | demagnetized and broken | 1.159 | 0.670 | 63.70 | 0.022 | 28.57 | 1.493 | 1.771 | 0.0204 | 31.854 | |
| whole | 0.297 | 1.02 | 19.9 | 0.078 | 8.1 | 0.3195 | 0.845 | 0.0129 | 9.277 | ||
| Leaching Conditions | Mass of Magnets for Leaching g | Mass of Metals in Leaching Solution g | Efficiency of Leaching % |
|---|---|---|---|
| 1 M H2SO4-RT | 13.149 | 9.510 | 70.50 |
| 1 M H2SO4–40 °C | 13.430 | 12.820 | 95.45 |
| 1 M H2SO4–60 °C | 13.880 | 13.335 | 96.07 |
| 2 M H2SO4-RT | 13.261 | 12.176 | 91.82 |
| 2 M H2SO4–40 °C | 12.864 | 9.786 | 76.07 |
| 2 M H2SO4–60 °C | 13.358 | 11.315 | 84.70 |
| 2 M HCl-RT | 13.631 | 11.861 | 87.01 |
| 2 M HCl–40 °C | 13.890 | 13.573 | 97.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klemettinen, A.; Żak, A.; Chojnacka, I.; Matuska, S.; Leśniewicz, A.; Wełna, M.; Adamski, Z.; Klemettinen, L.; Rycerz, L. Leaching of Rare Earth Elements from NdFeB Magnets without Mechanical Pretreatment by Sulfuric (H2SO4) and Hydrochloric (HCl) Acids. Minerals 2021, 11, 1374. https://doi.org/10.3390/min11121374
Klemettinen A, Żak A, Chojnacka I, Matuska S, Leśniewicz A, Wełna M, Adamski Z, Klemettinen L, Rycerz L. Leaching of Rare Earth Elements from NdFeB Magnets without Mechanical Pretreatment by Sulfuric (H2SO4) and Hydrochloric (HCl) Acids. Minerals. 2021; 11(12):1374. https://doi.org/10.3390/min11121374
Chicago/Turabian StyleKlemettinen, Anna, Andrzej Żak, Ida Chojnacka, Sabina Matuska, Anna Leśniewicz, Maja Wełna, Zbigniew Adamski, Lassi Klemettinen, and Leszek Rycerz. 2021. "Leaching of Rare Earth Elements from NdFeB Magnets without Mechanical Pretreatment by Sulfuric (H2SO4) and Hydrochloric (HCl) Acids" Minerals 11, no. 12: 1374. https://doi.org/10.3390/min11121374