Effect of Canal Bank Engineering Disturbance on Plant Communities: Analysis of Taxonomic and Functional Beta Diversity
Abstract
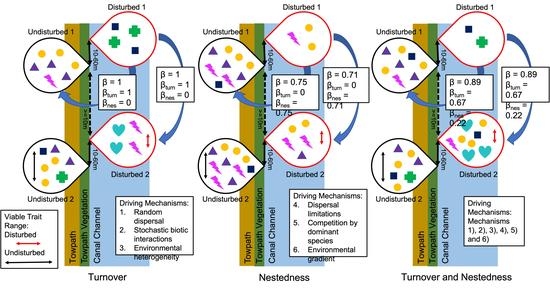
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Data Analysis
| Index | Partitioned Component | Formula | References | |
|---|---|---|---|---|
| Sørensen | βsor | Total Sørensen dissimilarity | [33,107] | |
| βsim | Simpson dissimilarity (turnover component of Sørensen) | [32,107,108] | ||
| βsne | Nestedness-resultant component of Sørensen | [48] | ||
| Jaccard | βjac | Total Jaccard dissimilarity | [31,107] | |
| βjtu | Turnover component of Jaccard | [98] | ||
| βjne | Nestedness-resultant component of Jaccard | [98] |
3. Results
3.1. Trait Validation and Overall Functional and Taxonomic Dissimilarity
3.2. Time since Disturbance
3.3. Dissimilarity within Each Disturbance Treatment
3.4. Effects of Environmental Variables
3.5. Invasion Status and Life-Form
4. Discussion
4.1. Taxonomic-β and Functional-β
4.2. Uncertainty of Functional Diversity
4.3. Disturbed versus Undisturbed β
4.4. Performance of β Indices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooke, R.L.; Martín-Duque, J.F. Land transformation by humans: A review. Geol. Soc. Am. 2012, 22, 4–10. Available online: https://www.geosociety.org/gsatoday/archive/22/12/abstract/i1052-5173-22-12-4.htm (accessed on 5 March 2023). [CrossRef]
- Woodwell, G. The Earth in Transition: Patterns and Processes of Biotic Impoverishment; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, Biodiversity, and Conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; White, P. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: San Diego, CA, USA; London, UK, 1985. [Google Scholar]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Labella, J.; de la Cruz, M.; Pescador, D.S.; Escudero, A. Individual species affect plant traits structure in their surroundings: Evidence of functional mechanisms of assembly. Oecologia 2016, 180, 975–987. [Google Scholar] [CrossRef]
- Götzenberger, L.; de Bello, F.; Bråthen, K.A.; Davison, J.; Dubuis, A.; Guisan, A.; Leps, J.; Lindborg, R.; Moora, M.; Pärtel, M.; et al. Ecological assembly rules in plant communities-approaches, patterns and prospects. Biol. Rev. 2011, 87, 111–127. [Google Scholar] [CrossRef]
- Schmidt, V.T.; Smith, K.F.; Melvin, D.W.; Amaral-Zettler, L.A. Community assembly of a euryhaline fish microbiome during salinity acclimation. Mol. Ecol. 2015, 24, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Troia, M.J.; Gido, K.B. Functional strategies drive community assembly of stream fishes along environmental gradients and across spatial scales. Oecologia 2015, 177, 545–559. [Google Scholar] [CrossRef]
- Kroll, A.J.; Verschuyl, J.; Giovanini, J.; Betts, M.G. Assembly dynamics of a forest bird community depend on disturbance intensity and foraging guild. J. Appl. Ecol. 2017, 54, 784–793. [Google Scholar] [CrossRef]
- Montaño-Centellas, F.A.; McCain, C.; Loiselle, B.A. Using functional and phylogenetic diversity to infer avian community assembly along elevational gradients. Glob. Ecol. Biogeogr. 2020, 29, 232–245. [Google Scholar] [CrossRef]
- Grime, J.P. Trait convergence and trait divergence in herbaceous plant communities: Mechanisms and consequences. J. Veg. Sci. 2006, 17, 255–260. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Crandall, R.M.; Jiménez, I. Disturbance alters beta-diversity but not the relative importance of community assembly mechanisms. J. Ecol. 2015, 103, 1291–1299. [Google Scholar] [CrossRef]
- Poos, M.S.; Walker, S.C.; Jackson, D.A. Functional-diversity indices can be driven by methodological choices and species richness. Ecology 2009, 90, 341–347. [Google Scholar] [CrossRef]
- McGill, B.J. Towards a unification of unified theories of biodiversity. Ecol. Lett. 2010, 13, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Sandel, B.; Kraft, N.J.B.; Carey, S. Characterizing scale-dependent community assembly using the functional-diversity–area relationship. Ecology 2013, 94, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Rosenblad, K.C.; Sax, D.F. A new framework for investigating biotic homogenization and exploring future trajectories: Oceanic island plant and bird assemblages as a case study. Ecography 2016, 39, 1040–1049. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Bromhead, J.C.; Beckwith, P. Environmental Dredging on the Birmingham Canals: Water Quality and Sediment Treatment. Water Environ. J. 1994, 8, 350–359. [Google Scholar] [CrossRef]
- Singh, S.P.; Tack, F.M.G.; Verloo, M.G. Extractability and bioavailability of heavy metals in surface soils derived from dredged sediments. Chem. Speciat. Bioavailab. 1996, 8, 105–110. [Google Scholar] [CrossRef]
- Hoosein, S. Soil Properties Affect Establishment of Invasive Species, Celastrus orbiculatus, in a Lower Hudson River Riparian Ecosystem. Master’s Thesis, State University of New York at Albany, Albany, NY, USA, 2016. [Google Scholar]
- Mayer, K.; Haeuser, E.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; et al. Naturalization of ornamental plant species in public green spaces and private gardens. Biol. Invasions 2017, 19, 3613–3627. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Toward a mechanistic understanding and prediction of biotic homogenization. Am. Nat. 2003, 162, 442–460. [Google Scholar] [CrossRef]
- Brice, M.-H.; Pellerin, S.; Poulin, M. Does urbanization lead to taxonomic and functional homogenization in riparian forests? Divers. Distrib. 2017, 23, 828–840. [Google Scholar] [CrossRef]
- Alignier, A.; Baudry, J. Is plant temporal beta diversity of field margins related to changes in management practices? Acta Oecologica 2016, 75, 1–7. [Google Scholar] [CrossRef]
- Freeman, J.E.; Kobziar, L.N.; Leone, E.H.; Williges, K. Drivers of plant functional group richness and beta diversity in fire-dependent pine savannas. Divers. Distrib. 2019, 25, 1024–1044. [Google Scholar] [CrossRef]
- Fukami, T.; Bezemer, M.T.; Mortimer, S.R.; Putten, W.H. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 2005, 8, 1283–1290. [Google Scholar] [CrossRef]
- Smart, S.M.; Thompson, K.; Marrs, R.H.; Le Duc, M.G.; Maskell, L.C.; Firbank, L.G. Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B Biol. Sci. 2006, 273, 2659–2665. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bremner, E.T. Assessing the impact of Impatiens glandulifera on riparian habitats: Partitioning diversity components following species removal. J. Appl. Ecol. 2006, 43, 43–50. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G.; Chave, J.; Terborgh, J.; Foster, R.B.; Nuñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Simpson, G.G. Mammals and the nature of continents. Am. J. Sci. 1943, 241, 1–31. [Google Scholar] [CrossRef]
- Sørensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. K. Dan. Vidensk. Selsk. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 280–338. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.-H.; Hsieh, T.C. Proposing a resolution to debates on diversity partitioning. Ecology 2012, 93, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How Should Beta-Diversity Inform Biodiversity Conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cortina, J.; Maestre, F.T.; Vallejo, R.; Baeza, M.J.; Valdecantos, A.; Pérez-Devesa, M. Ecosystem structure, function, and restoration success: Are they related? J. Nat. Conserv. 2006, 14, 152–160. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Bonser, S.P.; Morgan, J.W.; Aubin, I.; McNamara, S.; Vesk, P.A. What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob. Ecol. Biogeogr. 2010, 19, 423–431. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B Biol. Sci. 2011, 278, 877–884. [Google Scholar] [CrossRef]
- Laureto, L.M.O.; Cianciaruso, M.V.; Samia, D.S.M. Functional diversity: An overview of its history and applicability. Nat. Conserv. 2015, 13, 112–116. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Tuomisto, H. A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 2010, 33, 2–22. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Seidl, R. β-Diversity, Community Assembly, and Ecosystem Functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baetan, L.; Vangansbeke, P.; Hermy, M.; Peterken, G.; Vanhuyse, K.; Verheyen, K. Distinguishing between turnover and nested-ness in the quantification of biotic homogenization. Biodivers. Conserv. 2012, 21, 1399–1409. [Google Scholar] [CrossRef]
- Soininen, J.; Heino, J.; Wang, J.A. Meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob. Ecol. Biogeogr. 2018, 27, 96–109. [Google Scholar] [CrossRef]
- Devictor, V.; Mouillot, D.; Meynard, C.; Jiguet, F.; Thuiller, W.; Mouquet, N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world. Ecol. Lett. 2010, 13, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Church, S. The Basingstoke canal: A wildlife survival strategy. Ecos 1991, 12, 40–43. [Google Scholar]
- Stebbings, R.E. Bats in the Greywell Tunnell, Basingstoke Canal: An Assessment of the Bats in Greywell Tunnel and the Potential Effects on them of the Surrey and Hampshire Canal Society’s Restoration Proposals; Robert Stebbings Consultancy: Peterborough, UK, 1992. [Google Scholar]
- Pinkett, A.J. Basingstoke canal hydrological study: A water balance. J. Chart. Inst. Water Environ. Manag. 1995, 9, 376–384. [Google Scholar] [CrossRef]
- Morley, N.J.; Lewis, J.W. Anthropogenic pressure on a molluscan-trematode community over a long-term period in the Basingstoke canal, UK, and its implications for ecosystem health. EcoHealth 2006, 3, 269–280. [Google Scholar] [CrossRef]
- Ralphs, I.; Callegari, S. Survey of the Wetland Flora of the Basingstoke Canal: Report to the Basingstoke Canal Authority; Hampshire Biodiversity Information Centre: Basingstoke, UK, 2016. [Google Scholar]
- Tobler, W. A computer movie simulating urban growth in the Detroit region. Econ. Geogr. 1970, 46 (Suppl. 1), 234–240. [Google Scholar] [CrossRef]
- Jennings, S.B.; Brown, N.D.; Sheil, D. Assessing forest canopies and understorey illumination: Canopy closure, canopy cover and other measures. Forestry 1999, 72, 59–74. [Google Scholar] [CrossRef]
- Tichý, L.; Gap Light Analysis Mobile Application (Version 3.0). [Mobile App]. 2015. Available online: https://www.sci.muni.cz/botany/glama/GLAMA%20manual.pdf (accessed on 30 June 2020).
- Bianchi, S.; Cahalan, C.; Hale, S.; Gibbons, J.M. Rapid assessment of forest canopy and light regime using smartphone hemispherical photography. Ecol. Evol. 2017, 7, 10556–10566. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, L.; Korhonen, K.T.; Rautiainen, M.; Stenberg, P. Estimation of forest canopy cover: A comparison of Weld measurement techniques. Silva Fenn. 2006, 40, 577–588. [Google Scholar] [CrossRef]
- Public Interest Enterprises. Percentage Cover App (Version 1.0). [Mobile App]. 2017. Available online: https://apps.apple.com/us/app/percentage-cover/id1310190758 (accessed on 30 June 2020).
- Quintero, C.; Morales, C.L.; Aizen, M.A. Effects of anthropogenic habitat disturbance on local pollinator diversity and species turnover across a precipitation gradient. Biodivers. Conserv. 2009, 19, 257–274. [Google Scholar] [CrossRef]
- Bosch, J.; Retana, J.; Cerda, X. Flowering phenology, floral traits and pollinator composition in a herbaceous Mediterranean plant community. Oecologia 1997, 109, 583–591. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Kunin, W.E.; Garratt, M.P.D.; Potts, S.G.; Roy, H.E.; Andrews, C.; Jones, C.M.; Peyton, J.M.; Savage, J.; Harvey, M.C.; et al. Monitoring insect pollinators and flower visitation: The effectiveness and feasibility of different survey methods. Methods Ecol. Evol. 2019, 10, 2129–2140. [Google Scholar] [CrossRef]
- Carvell, C.; Isaac, N.J.B.; Jitlal, M.; Peyton, J.; Powney, G.D.; Roy, D.B.; Vanbergen, A.J.; O’Connor, R.; Jones, C.; Kunin, B.; et al. Design and Testing of a National Pollinator and Pollination Monitoring Framework; Centre for Ecology & Hydrology: Wallingford, UK.
- Cane, J.H.; Tepedino, V.J. Causes and extent of declines among native North American invertebrate pollinators: Detection, evidence, and consequences. Conserv. Ecol. 2001, 5, 1. Available online: http://www.consecol.org/vol5/iss1/art1/ (accessed on 5 March 2023). [CrossRef]
- Nowakowski, M.; Pywell, R.F. Habitat Creation and Management for Pollinators; Centre for Ecology and Hydrology: Wallingford, UK, 2016. [Google Scholar]
- Jones, H.G. Visual Flora. 2019. Available online: https://visual-flora.org.uk/index.html (accessed on 30 June 2020).
- Glority Global Group Ltd. PictureThis Mobile Application (2.6.4). [PictureThis]. 2020. Available online: https://www.picturethisai.com/?fbclid=IwAR08UPTQTn7NJX0Y0PVscR92J_KzHtFznfztYFf9KuEHPjCiHTG49lpxeQQ (accessed on 30 June 2020).
- Hall, C.; Groome, G. 2012 Survey of the wetland flora of the Basingstoke Canal. In Report to Basingstoke Canal Authority; Basingstoke Canal Authority: Mychett, UK, 2013. [Google Scholar]
- Botanical Society of Britain and Ireland (BSBI). Online Atlas of British and Irish Flora. 2020. Available online: https://www.brc.ac.uk/plantatlas/ (accessed on 30 June 2020).
- Kumar, N.; Belhumeur, P.N.; Biswas, A.; Jacobs, D.W.; John Kress, W.; Lopez, I.C.; Soares, J.V.B. Leafsnap: A Computer Vision System for Automatic Plant Species Identification. In Lecture Notes in Computer Science, Computer Vision–ECCV 2012: 12th European Conference on Computer Vision, Florence, Italy, 7–13 October 2012; Fitzgibbon, A., Lazebnik, S., Perona, P., Sato, Y., Schmid, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7573. [Google Scholar] [CrossRef]
- Bonnet, P.; Goëau, H.; Hang, S.T.; Lasseck, M.; Šulc, M.; Malécot, V.; Jauzein, P.; Melet, J.-C.; You, C.; Joly, A. Plant Identification: Experts vs. Machines in the Era of Deep Learning. In Multimedia Tools and Applications for Environmental and Biodiversity Informatics; Multimedia Systems and Applications; Joly, A., Vrochidis, S., Karatzas, K., Karppinen, A., Bonnet, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 131–149. [Google Scholar] [CrossRef]
- Weiher, E.; van der Werf, A.; Thompson, K.; Roderick, M.; Garnier, E.; Eriksson, O. Challenging Theophrastus: A common core list of plant traits for functional ecology. J. Veg. Sci. 1999, 10, 609–620. [Google Scholar] [CrossRef]
- Latzel, V.; Mihulka, S.; Klimešová, J. Plant traits and regeneration of urban plant communities after disturbance: Does the bud bank play any role? Appl. Veg. Sci. 2008, 11, 387–394. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Gray, A.; Vanbergen, A.J.; Bergès, L.; Bohner, A.; Brooker, R.W.; De Bruyn, L.; De Cinti, B.; Dirnböck, T.; Grandin, U.; et al. Functional traits and local environment predict vegetation responses to disturbance: A pan-European multi-site experiment. J. Ecol. 2011, 99, 777–787. [Google Scholar] [CrossRef]
- Herben, T.; Klimešová, J.; Chytrý, M. Effects of disturbance frequency and severity on plant traits: An assessment across a temperate flora. Funct. Ecol. 2018, 32, 799–808. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villéger, S.; De Bortoli, J.; Leprieur, F.; Logez, M.; Henriques-Silva, R.; betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5.2. [Software]. 2020. Available online: https://CRAN.R-project.org/package=betapart (accessed on 4 October 2020).
- Laughlin, D.C. The intrinsic dimensionality of plant traits and its relevance to community assembly. J. Ecol. 2014, 102, 186–193. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Leppert, J.J.; Moore, M.M.; Sieg, C.H. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2009, 24, 493–501. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Fitter, A.H.; Peat, H.J. The Ecological Flora Database. J. Ecol. 1994, 82, 415–425. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.; Knevel, I.; Bakker, J.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Unwin Hyman: London, UK, 1988. [Google Scholar]
- Fern, K. Plants for a Future: Edible and Useful Plants for a Healthier World; Permanent Publications: Hampshire, UK, 1997; ISBN 1-85623-011-2. [Google Scholar]
- Májeková, M.; Paal, T.; Plowman, N.S.; Bryndová, M.; Kasari, L.; Norberg, A.; Weiss, M.; Bishop, T.R.; Luke, S.H.; Sam, K.; et al. Evaluating Functional Diversity: Missing Trait Data and the Importance of Species Abundance Structure and Data Transformation. PLoS ONE 2016, 11, e0149270. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Da Silva, F.C.G. Using Plant Functional Traits to Assess Ecosystem Processes and Community Dynamics in Lowland Fens: Understanding the Efficacy and Applicability of a Trait-Based Approach to Plant Ecology. Ph.D. Thesis, Kingston University, London, UK, 2017. [Google Scholar]
- Rejmánek, M.; Richardson, D.M. What attributes make some plant species more invasive? Ecology 1996, 77, 1655–1661. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 March 2023).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 5 March 2023).
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Xing, D.; He, F. Analytical models for β-diversity and the power-law scaling of β-deviation. Methods Ecol. Evol. 2020, 12, 405–414. [Google Scholar] [CrossRef]
- Gallagher, E.D. COMPAH Documentation; University of Massachusetts: Boston, MA, USA, 1999. [Google Scholar]
- McCune, B.; Grace, J. Analysis of Ecological Communities; MJM Software: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Barwell, L.J.; Isaac, N.J.B.; Kunin, W.E. Measuring β-diversity with species abundance data. J. Anim. Ecol. 2015, 84, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hervé, M.; RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package (Version 0.9-79). [Software]. 2021. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 21 February 2021).
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd English Edition; Elsevier Science BV: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Oksanen, J.; Blanchet, F.J.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; Wagner, H.; vegan: Community Ecology Package. R package (Version 2.5-7). [Software]. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 February 2021).
- Maria, E.; Budiman, E.; Haviluddin, H.; Taruk, M. Measure distance locating nearest public facilities using Haversine and Euclidean Methods. IOP-J. Phys. Conf. Ser. 2020, 1450, 012080. [Google Scholar] [CrossRef]
- Swenson, N.G. Null Models. In Functional and Phylogenetic Ecology in R. Use R! Springer: New York, NY, USA, 2014; pp. 109–146. [Google Scholar] [CrossRef]
- Götzenberger, L.; Botta-Dukát, Z.; Lepš, J.; Pärtel, M.; Zobel, M.; de Bello, F. Which randomizations detect convergence and divergence in trait-based community assembly? A test of commonly used null models. J. Veg. Sci. 2016, 27, 1275–1287. [Google Scholar] [CrossRef]
- Heydari, M.; Omidipour, R.; Abedi, M.; Baskin, C. Effects of fire disturbance on alpha and beta diversity and on beta diversity components of soil seed banks and above ground vegetation. Plant Ecol. Evol. 2017, 150, 247–256. [Google Scholar] [CrossRef]
- Daniel, J.; Gleason, J.E.; Cottenie, K.; Rooney, R.C. Stochastic and deterministic processes drive wetland community assembly across a gradient of environmental filtering. Oikos 2019, 128, 1158–1169. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Jeppesen, E.; Ge, D.; Li, W.; Zou, D.; Huang, Z.; Wu, A.; Liu, Q. Local and regional drivers of turnover and nestedness components of species and functional beta diversity in lake macrophyte communities in China. Sci. Total Environ. 2019, 687, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Veech, J.A. Analysing patterns of species diversity as departures from random expectations. Oikos 2005, 108, 149–155. [Google Scholar] [CrossRef]
- Mi, X.; Swenson, N.G.; Jia, Q.; Rao, M.; Feng, G.; Ren, H.; Bebber, D.P.; Ma, K. Stochastic assembly in a subtropical forest chronosequence: Evidence from contrasting changes of species, phylogenetic and functional dissimilarity over succession. Sci. Rep. 2016, 6, 32596. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Natuhara, Y. Effect of Anthropogenic Disturbance on Floristic Homogenization in the Floodplain Landscape: Insights from the Taxonomic and Functional Perspectives. Forests 2020, 11, 1036. [Google Scholar] [CrossRef]
- Castro, S.A.; Rojas, P.; Vila, I.; Habit, E.; Pizarro-Konczak, J.; Abades, S.; Jaksic, F.M. Partitioning β-diversity reveals that invasions and extinctions promote the biotic homogenization of Chilean freshwater fish fauna. PLoS ONE 2020, 15, e0238767. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Low impact of disturbance on ecological success of invasive tree and shrub species in temperate forests. Plant Ecol. 2018, 219, 1369–1380. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Lilley, P.L.; Vellend, M. Negative native–exotic diversity relationship in oak savannas explained by human influence and climate. Oikos 2009, 118, 1373–1382. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Yelenik, S.G.; Colman, B.P.; Levine, J.M. California annual grass invaders: The drivers or passengers of change? J. Ecol. 2010, 98, 1147–1156. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Villarreal, D.; Lekberg, Y.; Cock, M.C.; Eren, Ö.; Hierro, J.L. The fluctuating resource hypothesis explains invasibility, but not exotic advantage following disturbance. Ecology 2018, 99, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Vacher, K.A.; Killingbeck, K.T.; August, P.V. Is the relative abundance of non-native species an integrated measure of anthropogenic disturbance? Landsc. Ecol. 2007, 22, 821–835. [Google Scholar] [CrossRef]
- Lozon, J.D.; MacIsaac, H.J. Biological invasions: Are they dependent on disturbance? Environ. Rev. 1997, 5, 131–144. [Google Scholar] [CrossRef]
- Bottollier-Curtet, M.; Planty-Tabacchi, A.-M.; Tabacchi, E. Competition between young exotic invasive and native dominant plant species: Implications for invasions within riparian areas. J. Veg. Sci. 2013, 24, 1033–1042. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Pauchard, A.; Lenoir, J.; Nuñez, M.A.; Geron, C.; Ven, A.; Bravo-Monasterio, P.; Teneb, E.; Nijs, I.; Milbau, A. Disturbance is the key to plant invasions in cold environments. Proc. Natl. Acad. Sci. USA 2016, 113, 14061–14066. [Google Scholar] [CrossRef]
- Catano, C.P.; Dickson, T.L.; Myers, J.A. Dispersal and neutral sampling mediate contingent effects of disturbance on plant beta-diversity: A meta-analysis. Ecol. Lett. 2017, 20, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Segre, H.; Ron, R.; De Malach, N.; Henkin, Z.; Mandel, M.; Kadmon, R. Competitive exclusion, beta diversity, and deterministic vs. stochastic drivers of community assembly. Ecol. Lett. 2014, 17, 1400–1408. [Google Scholar] [CrossRef]
- del Moral, R.; Wood, D.M.; Titus, J.H. Proximity, Microsites, and Biotic Interactions During Early Succession. In Ecological Responses to the 1980 Eruption of Mount St. Helens; Dale, V.H., Swanson, F.J., Crisafulli, C.M., Eds.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Naaf, T.; Wulf, M. Does taxonomic homogenization imply functional homogenization in temperate forest herb layer communities? Plant Ecol. 2012, 213, 431–443. [Google Scholar] [CrossRef]
- Knapp, S.; Kühn, I.; Schweiger, O.; Klotz, S. Challenging urban species diversity: Contrasting phylogenetic patterns across plant functional groups in Germany. Ecol. Lett. 2008, 11, 1054–1064. [Google Scholar] [CrossRef]
- Vitorino Júnior, O.B.; Fernandes, R.; Agostinho, C.S.; Pelicice, F.M. Riverine networks constrain β-diversity patterns among fish assemblages in a large Neotropical river. Freshw. Biol. 2016, 61, 1733–1745. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Terlizzi, A. Nestedness and turnover unveil inverse spatial patterns of compositional and functional β-diversity at varying depth in marine benthos. Divers. Distrib. 2020, 26, 743–757. [Google Scholar] [CrossRef]
- Aspin, T.W.H.; Matthews, T.J.; Khamis, K.; Milner, A.M.; Wang, Z.; O’Callaghan, M.J.; Ledger, M.E. Drought intensification drives turnover of structure and function in stream invertebrate communities. Ecography 2018, 41, 1992–2004. [Google Scholar] [CrossRef]
- Siefert, A.; Ravenscroft, C.; Weiser, M.D.; Swenson, N.G. Functional beta-diversity patterns reveal deterministic community assembly processes in eastern North American trees. Glob. Ecol. Biogeogr. 2012, 22, 682–691. [Google Scholar] [CrossRef]
- Pinto-Ledezma, J.N.; Larkin, D.J.; Cavender-Bares, J. Patterns of Beta Diversity of Vascular Plants and Their Correspondence with Biome Boundaries Across North America. Front. Ecol. Evol. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Lenssen, J.P.M.; Van de Steeg, H.; De Kroon, H. Does Disturbance Favour Weak Competitors? Mechanisms of Changing Plant Abundance after Flooding. J. Veg. Sci. 2004, 15, 305–314. Available online: http://www.jstor.org/stable/3236470 (accessed on 5 March 2023). [CrossRef]
- Stępień, E.; Zawal, A.; Buczyński, P.; Buczyńska, E.; Szenejko, M. Effects of dredging on the vegetation in a small lowland river. PeerJ 2019, 7, e6282. [Google Scholar] [CrossRef]
- Lefcheck, J.; Bastazini, V.; Griffin, J. Choosing and using multiple traits in functional diversity research. Environ. Conserv. 2015, 42, 104–107. [Google Scholar] [CrossRef]
- Butler, E.E.; Datta, A.; Flores-Moreno, H.; Chen, M.; Wythers, K.R.; Fazayeli, F.; Banerjee, A.; Atkin, O.K.; Kattge, J.; Amiaud, B.; et al. Mapping local and global variability in plant trait distributions. Proc. Natl. Acad. Sci. USA 2017, 114, E10937–E10946. [Google Scholar] [CrossRef]
- He, D.; Chen, Y.; Zhao, K.; Cornelissen, J.H.C.; Chu, C. Intra- and interspecific trait variations reveal functional relationships between specific leaf area and soil niche within a subtropical forest. Ann. Bot. 2018, 121, 1173–1182. [Google Scholar] [CrossRef]
- Kazakou, E.; Violle, C.; Roumet, C.; Navas, M.-L.; Vile, D.; Kattge, J.; Garnier, E. Are trait-based species rankings consistent across data sets and spatial scales? J. Veg. Sci. 2013, 25, 235–247. [Google Scholar] [CrossRef]
- Sandel, B.; Gutiérrez, A.G.; Reich, P.B.; Schrodt, F.; Dickie, J.; Kattge, J. Estimating the missing species bias in plant trait measurements. J. Veg. Sci. 2015, 26, 828–838. [Google Scholar] [CrossRef]
- MacArthur, R.H. Population Ecology of Some Warblers of Northeastern Coniferous Forests. Ecology 1958, 39, 599–619. [Google Scholar] [CrossRef]
- Stoll, P.; Weiner, J. A neighborhood view of interactions among individual plants. In The Geometry of Ecological Interactions-Simplifying Spatial Complexity; Dieckmann, U., Law, R., Metz, J.A.J., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 11–27. [Google Scholar]
- Cook, S.R.; Parker, A. Geochemical changes to dredged canal sediments following land spreading: A review. Land Contam. Reclam. 2003, 11, 405–410. [Google Scholar] [CrossRef]
- Torrance, K.; Lord, R.; Geochemical Aspects to Reusing Dredged Canal Sediment. Society for Environmental Geochemistry and Health: Members Blog. [Blog] 9 July. 2020. Available online: https://segh.net/f/geochemical-aspects-to-reusing-dredged-canal-sediment (accessed on 23 February 2021).
- Najeeb, U.; Ahmad, W.; Zia, M.H.; Zaffar, M.; Zhou, W. Enhancing the lead phytostabilization in wetland plant Juncus effusus L. through somaclonal manipulation and EDTA enrichment. Arab. J. Chem. 2017, 10, S3310–S3317. [Google Scholar] [CrossRef]
- Chalmandrier, L.; Münkemüller, T.; Gallien, L.; de Bello, F.; Mazel, F.; Lavergne, S.; Thuiller, W. A family of null models to distinguish between environmental filtering and biotic interactions in functional diversity patterns. J. Veg. Sci. 2013, 24, 853–864. [Google Scholar] [CrossRef]
- Hao, M.; Corral-Rivas, J.J.; González-Elizondo, M.S.; Ganeshaiah, K.N.; Nava-Miranda, M.G.; Zhang, C.; Zhao, X.; Von Gadow, K. Assessing biological dissimilarities between five forest communities. For. Ecosyst. 2019, 6, 30. [Google Scholar] [CrossRef]
- Knijnenburg, T.A.; Wessels, L.F.; Reinders, M.J.; Shmulevich, I. Fewer permutations, more accurate P-values. Bioinformatics 2009, 25, i161–i168. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Dushoff, J.; Kain, M.P.; Bolker, B.M. I can see clearly now: Reinterpreting statistical significance. Methods Ecol. Evol. 2019, 10, 756–759. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a world beyond “P<0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef]
- Yoccoz, N. Use, Overuse, and Misuse of Significance Tests in Evolutionary Biology and Ecology. Bull. Ecol. Soc. Am. 1991, 72, 106–111. Available online: http://www.jstor.org/stable/20167258 (accessed on 5 March 2023).
- Barbet-Massin, M.; Jetz, W. The effect of range changes on the functional turnover, structure and diversity of bird assemblages under future climate scenarios. Glob. Change Biol. 2015, 21, 2917–2928. [Google Scholar] [CrossRef]
- Barnagaud, J.-Y.; Kissling, W.D.; Tsirogiannis, C.; Fisikopoulos, V.; Villéger, S.; Sekercioglu, C.H.; Svenning, J.-C. Biogeographical, environmental and anthropogenic determinants of global patterns in bird taxonomic and trait turnover. Glob. Ecol. Biogeogr. 2017, 26, 1190–1200. [Google Scholar] [CrossRef]
- Braghin, L.S.M.; Almeida, B.A.; Amaral, D.C.; Canella, T.F.; Gimenez, B.C.G.; Bonecker, C.C. Effects of dams decrease zooplankton functional β-diversity in river-associated lakes. Freshw. Biol. 2018, 63, 721–730. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Hattab, T.; Decocq, G. Do drivers of forestry vehicles also drive herb layer changes (1970–2015) in a temperate forest with contrasting habitat and management conditions? J. Ecol. 2018, 107, 1439–1456. [Google Scholar] [CrossRef]
- Crabot, J.; Polášek, M.; Launay, B.; Pařil, P.; Datry, T. Drying in newly intermittent rivers leads to higher variability of invertebrate communities. Freshw. Biol. 2021, 66, 730–744. [Google Scholar] [CrossRef]
- Damgaard, C. Estimating mean plant cover from different types of cover data: A coherent statistical framework. Ecosphere 2014, 5, 20. [Google Scholar] [CrossRef]
- Fournier, B.; Frey, D.; Moretti, M. The origin of urban communities: From the regional species pool to community assemblages in city. J. Biogeogr. 2020, 47, 615–629. [Google Scholar] [CrossRef]
- Leigh, C.; Aspin, T.W.H.; Matthews, T.J.; Rolls, R.J.; Ledger, M.E. Drought alters the functional stability of stream invertebrate communities through time. J. Biogeogr. 2019, 46, 1988–2000. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H. Effects of loss of lateral hydrological connectivity on fish functional diversity. Conserv. Biol. 2018, 32, 1336–1345. [Google Scholar] [CrossRef]
- Mathers, K.L.; White, J.C.; Guareschi, S.; Hill, M.J.; Heino, J.; Chadd, R. Invasive crayfish alter the long-term functional biodiversity of lotic macroinvertebrate communities. Funct. Ecol. 2020, 34, 2350–2361. [Google Scholar] [CrossRef]
- Milberg, P.; Bergstedt, J.; Fridman, J.; Odell, G.; Westerberg, L. Systematic and random variation vegetation monitoring data. J. Veg. Sci. 2008, 19, 633–644. [Google Scholar] [CrossRef]
- Nielsen, A.; Totland, Ø. Structural properties of mutualistic networks withstand habitat degradation while species functional roles might change. Oikos 2014, 123, 323–333. [Google Scholar] [CrossRef]
- Pereyra, L.C.; Akmentins, M.S.; Vaira, M.; Moreno, C.E. Disentangling the multiple components of anuran diversity associated to different land-uses in Yungas forests, Argentina. Anim. Conserv. 2018, 21, 396–404. [Google Scholar] [CrossRef]
- Ringvall, A.; Petersson, H.; Ståhl, G.; Lämås, T. Surveyor consistency in presence/absence sampling for monitoring vegetation in a boreal forest. For. Ecol. Manag. 2005, 212, 109–117. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Booth, D.T. Measuring Plant Cover in Sagebrush Steppe Rangelands: A Comparison of Methods. Environ. Manag. 2006, 37, 703–711. [Google Scholar] [CrossRef]
- Sykes, J.M.; Horrill, A.D.; Mountford, M.D. Use of visual cover assessments as quantitative estimators of some British woodland taxa. J. Ecol. 1983, 71, 437–450. [Google Scholar] [CrossRef]







| Variable | Definition | Collection Method | Method Reference | Notes |
|---|---|---|---|---|
| Canopy Closure | Percent of sky hemisphere occupied by vegetation, viewed from a single point [58]. | GLAMA 3.0 App and external Mpow 180 Degree Supreme Fisheye Lens | [59,60] | Canopy Closure and Modified Canopy Closure with/without a 40° horizon mask (to control for 150° cropping by smartphone screen). |
| Canopy Cover | Percent of plot area occupied by the vertical projection of tree crowns [61] | % Canopy Cover App and built-in smartphone camera | [62] | Photo always perpendicular to canal bank, photo converted to binary and cut level designated by eye. |
| Inflorescence Abundance | A single inflorescence is a complete flower head irrespective of morphology (e.g., singular/clustered) | Visual assessment | [63] | |
| Inflorescence Type | Corolla morphology defined as either “open”, “tubular” or “closed”. | Visual assessment | [64] | |
| Bare ground % | Percent of 2 m × 0.5 m plot not covered by vegetation. | Visual estimation | N/A | |
| Pollinator Abundance | Insects touching the reproductive parts of a plant within the plot area; pollinators grouped into morphological categories to avoid misidentification. Species accumulation curve was used to identify 10 min as the optimum sampling time. | Followed methodology of UK Pollinator Monitoring scheme with added morphological categories of flies > 3 mm and flies < 3 mm. | [65,66] | From 9 am to 6 pm [63] cloudy days ≥ 15 °C, sunny days ≥ 17 °C, no wind and no rain. |
| Pollinator Habitats | Y/N recorded for whether there was dead wood, brush piles or patchy, sheltered ground. | Visual assessment | [67,68] | Pollinator habitat production must take into account all life stages. Assessment within 15 m of each study site. |
| Tree Cover Type | Species composing the overhead tree canopy were identified and recorded. | PictureThis (2.6.4) App and Visual-flora online key | [69,70] | Only the presence/absence of tree species, not relative abundances. |
| Aquatic Macrophytes | Presence/absence of emergent and/or submerged macrophytes directly adjacent to the study site. | Visual assessment | N/A |
| Trait | Ecological Implications | References | Justification for Inclusion/Exclusion |
|---|---|---|---|
| Specific leaf area (SLA) | Competitive ability, stress tolerance and potential relative growth rate. Correlated negatively with leaf longevity and positively with leaf nitrogen. | [75,83,91,92,93] | Included: relatively stable traits and all components of the L-H-S spectrum (Westoby, 1998 [81]) informative of the C-S-R spectrum important in disturbance ecology (Grime, 1977 [90]). |
| Generative plant height | Competitive ability, potential lifespan, fecundity and whether a plant can achieve reproductive height between disturbance events. Closely related to aboveground biomass. | ||
| Seed (or dispersule) mass | Dispersal capacity, establishment ability, longevity in seed bank and fecundity. | ||
| Plant life form and plant growth form | Establishment ability, invasiveness and disturbance response. | [92,94] | Rejected: categorical traits cannot be included in calculations for partitioning pairwise functional beta diversity [95]. |
| Leaf life span | Stress tolerance, trade-off between defence and growth rate, and leaf litter decomposition rate. | [92] | |
| Resprouting capacity after major disturbance | Stress tolerance, likelihood of major disturbance events. Negatively correlated with reproduction and growth rate. | [83] | |
| Life history | Disturbance response, establishment and invasiveness. | [83] | |
| Invasiveness | Establishment; disturbance tolerance. | [76] | |
| Leaf nitrogen and leaf phosphorus | Potential relative growth rate. Often correlated with SLA, nutritional quality for consumers and mass-based maximum photosynthetic rate. | [83,93] | Rejected: high intraspecific variation depending on soil types/nutrient loading [83]—data from global TRY database unlikely to be suitable for this study scale. |
| Leaf water content | Water stress tolerance, relative growth rate and linked to salinity tolerance. | [75,83] | Rejected: varies diurnally and with relative soil moisture [83] |
| Partitioned Component | Fixed Factors | df | Error df | F | p | |
|---|---|---|---|---|---|---|
| Jaccard Turnover | Year | 1 | 108 | 0.595 | 0.442 | |
| Beta type | 1 | 108 | 29.028 | 0.000 | *** | |
| Year: Beta type | 1 | 108 | 1.138 | 0.288 | ||
| Jaccard Nestedness | Year | 1 | 108 | 1.133 | 0.290 | |
| Beta type | 1 | 108 | 12.891 | 0.000 | *** | |
| Year: Beta type | 1 | 108 | 4.928 | 0.029 | * | |
| Jaccard Total | Year | 1 | 108 | 0.022 | 0.883 | |
| Beta type | 1 | 108 | 33.621 | 0.000 | *** | |
| Year: Beta type | 1 | 108 | 1.289 | 0.259 | ||
| Sørensen Turnover | Year | 1 | 108 | 0.627 | 0.430 | |
| Beta type | 1 | 108 | 27.881 | 0.000 | *** | |
| Year: Beta type | 1 | 108 | 1.224 | 0.271 | ||
| Sørensen Nestedness | Year | 1 | 108 | 1.228 | 0.270 | |
| Beta type | 1 | 108 | 6.683 | 0.011 | * | |
| Year: Beta type | 1 | 108 | 6.487 | 0.012 | * | |
| Sørensen Total | Year | 1 | 108 | 0.013 | 0.910 | |
| Beta type | 1 | 108 | 32.118 | 0.000 | *** | |
| Year: Beta type | 1 | 108 | 0.952 | 0.331 |
| Treatment | Mean Disturbed | SE | Mean Undisturbed | SE |
|---|---|---|---|---|
| Jaccard/Ruzicka Turnover | 0.732 | 0.003 | 0.735 | 0.003 |
| Jaccard/Ruzicka Nestedness | 0.569 | 0.005 | 0.508 | 0.005 |
| Jaccard/Ruzicka Total dissimilarity | 0.888 | 0.002 | 0.834 | 0.002 |
| Sørensen/Bray–Curtis Turnover | 0.297 | 0.004 | 0.228 | 0.003 |
| Sørensen/Bray–Curtis Nestedness | 0.186 | 0.003 | 0.105 | 0.002 |
| Sørensen/Bray–Curtis Total dissimilarity | 0.819 | 0.002 | 0.734 | 0.002 |
| Beta Diversity Type | Mean Taxonomic | SE | Mean Functional | SE |
| Jaccard/Ruzicka Turnover | 0.588 | 0.003 | 0.879 | 0.001 |
| Jaccard/Ruzicka Nestedness | 0.210 | 0.003 | 0.867 | 0.002 |
| Jaccard/Ruzicka Total dissimilarity | 0.798 | 0.002 | 0.923 | 0.001 |
| Sørensen/Bray–Curtis Turnover | 0.460 | 0.003 | 0.066 | 0.001 |
| Sørensen/Bray–Curtis Nestedness | 0.226 | 0.003 | 0.066 | 0.001 |
| Sørensen/Bray–Curtis Total dissimilarity | 0.686 | 0.002 | 0.867 | 0.002 |
| Partitioned Component | Fixed Factors | df | Error | F-Value | p-Value | Sig. |
|---|---|---|---|---|---|---|
| Jaccard/Ruzicka Turnover | Treatment | 1 | 12,316 | 0.574 | 0.455 | |
| Beta type | 1 | 12,316 | 7099.02 | 0.000 | *** | |
| Treatment: Beta type | 1 | 12,316 | 30.142 | 0.000 | *** | |
| Jaccard/Ruzicka Nestedness | Treatment | 1 | 12,316 | 387.044 | 0.001 | *** |
| Beta type | 1 | 12,316 | 44,128.5 | 0.001 | *** | |
| Treatment: Beta type | 1 | 12,316 | 30.753 | 0.001 | *** | |
| Jaccard/Ruzicka Total | Treatment | 1 | 12,316 | 733.303 | 0.001 | *** |
| Beta type | 1 | 12,316 | 3925.17 | 0.001 | *** | |
| Treatment: Beta type | 1 | 12,316 | 10.496 | 0.003 | ** | |
| Sørensen/Bray–Curtis | Treatment | 1 | 12,316 | 387.840 | 0.001 | *** |
| Beta type | 1 | 12,316 | 12,684.1 | 0.001 | *** | |
| Treatment: Beta type | 1 | 12,316 | 103.950 | 0.001 | *** | |
| Sørensen/Bray–Curtis | Treatment | 1 | 12,316 | 728.272 | 0.001 | *** |
| Nestedness | Beta type | 1 | 12,316 | 2868.25 | 0.001 | *** |
| Treatment: Beta type | 1 | 12,316 | 62.265 | 0.001 | *** | |
| Sørensen/Bray–Curtis Total | Treatment | 1 | 12,316 | 926.226 | 0.001 | *** |
| Beta type | 1 | 12,316 | 4200.59 | 0.001 | *** | |
| Treatment: Beta type | 1 | 12,316 | 4.479 | 0.034 | * |
| Dissimilarity Measure | Notation | Canopy Cover | Geographic Distance | ||
|---|---|---|---|---|---|
| Functional Diversity | Mantel’s r | p | Mantel’s r | p | |
| Total Sørensen dissimilarity | βsor | −0.07253 | 0.989 | 0.007229 | 0.358 |
| Simpson (Turnover) | βsim | −0.05412 | 0.885 | 0.06611 * | 0.018 |
| Nestedness (Sørensen) | Βsne | 0.01832 | 0.333 | −0.06292 | 0.982 |
| Total Jaccard dissimilarity | βjac | −0.06862 | 0.990 | 0.006352 | 0.371 |
| Turnover (Jaccard) | βjtu | −0.05653 | 0.899 | 0.06962 * | 0.014 |
| Nestedness (Jaccard) | βjne | 0.03228 | 0.217 | −0.06837 | 0.991 |
| Taxonomic (abundance) diversity | |||||
| Total Bray–Curtis dissimilarity | βbray | −0.02003 | 0.708 | 0.05349 * | 0.049 |
| Turnover equivalent (Bray–Curtis) | βbray_bal | 0.03625 | 0.111 | 0.08619 *** | 0.000 |
| Nestedness equivalent (Bray–Curtis) | βbray_gra | −0.05267 | 0.921 | −0.04825 | 0.973 |
| Total Ruzicka dissimilarity | βruz | −0.0163 | 0.674 | 0.04805 * | 0.036 |
| Turnover equivalent (Ruzicka) | βruz_bal | 0.03711 | 0.095 | 0.07966 *** | 0.000 |
| Nestedness equivalent (Ruzicka) | βruz_gra | −0.04767 | 0.921 | −0.06576 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugh, B.E.; Field, R. Effect of Canal Bank Engineering Disturbance on Plant Communities: Analysis of Taxonomic and Functional Beta Diversity. Land 2023, 12, 1090. https://doi.org/10.3390/land12051090
Pugh BE, Field R. Effect of Canal Bank Engineering Disturbance on Plant Communities: Analysis of Taxonomic and Functional Beta Diversity. Land. 2023; 12(5):1090. https://doi.org/10.3390/land12051090
Chicago/Turabian StylePugh, Brittany E., and Richard Field. 2023. "Effect of Canal Bank Engineering Disturbance on Plant Communities: Analysis of Taxonomic and Functional Beta Diversity" Land 12, no. 5: 1090. https://doi.org/10.3390/land12051090