Genes Involved in DNA Damage Cell Pathways and Health of the Oldest-Old (85+)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study and Participants
2.2. Selection of Genetic Loci and Genotyping Method
2.3. Questionnaire
2.4. Data Analysis
3. Results
3.1. Subjective General Health
3.2. Objective General Health
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
- -
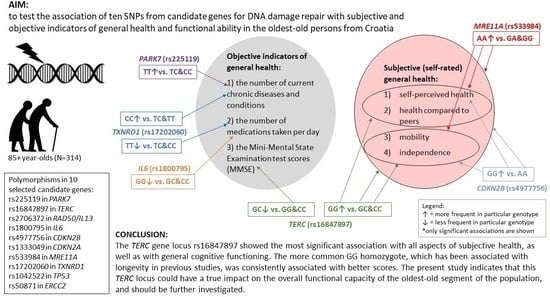
- The examined ten genetic loci involved in the cellular pathways of DNA damage repair showed a more significant association with the self-rated health and functional ability scores than with the number of diagnosed diseases or prescribed drugs.
- -
- Subjective and objective indicators of general health were almost entirely related to different genetic loci. Also, general cognitive function, as assessed by the MMSE score, was matched with subjective general health variables.
- -
- rs16847897 in the TERC gene showed the most significant relationships with both quantitative and qualitative subjective general health variables and was associated with all three aspects of self-assessments: health, mobility, and independence (as well as with the MMSE score). The more frequent GG homozygote of this genetic locus, which has been associated with longevity in previous studies, was consistently related to better scores.
- -
- The most significant relationship found between the binarised variable of functional ability (sum of self-rated mobility and independence) and rs16847897 in the TERC gene remained when an additional eight variables were included in the multivariate logistic regression model, indicating that the TERC locus investigated here might have a true impact on the overall vitality of the oldest-old people.
- -
- For a firm conclusion on the connection of vitality and health with cellular ageing, a much more extensive experimental design is needed (more SNP loci such as in GWAS), and the results need to be confirmed in other populations.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.A.; Crawford, J.D.; Riches, J.C.; Kochan, N.A.; Chander, R.J.; Mather, K.A.; Sachdev, P.S.; Henry, J.D. Aging Is Associated with Multidirectional Changes in Social Cognition: Findings from an Adult Life-Span Sample Ranging from 18 to 101 Years. J. Gerontol. Ser. B 2023, 78, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef]
- He, W.; Goodkind, D.; Kowal, P. An Aging World 2015: International Population Reports; U.S. Government Publishing Office: Washington, DC, USA, 2016; pp. 3–13. [Google Scholar]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef] [PubMed]
- Vijg, J.; Suh, Y. Genome Instability and Aging. Annu. Rev. Physiol. 2013, 75, 645–668. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and Telomere Shortening: Insights from Cellular Mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Press, M.; Jung, T.; König, J.; Grune, T.; Höhn, A. Protein Aggregates and Proteostasis in Aging: Amylin and β-Cell Function. Mech. Ageing Dev. 2019, 177, 46–54. [Google Scholar] [CrossRef]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic Clock: A Promising Biomarker and Practical Tool in Aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef]
- Huang, R.-X.; Zhou, P.-K. DNA Damage Response Signaling Pathways and Targets for Radiotherapy Sensitization in Cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Carney, J.P.; Maser, R.S.; Olivares, H.; Davis, E.M.; Le Beau, M.; Yates, J.R.; Hays, L.; Morgan, W.F.; Petrini, J.H. The HMre11/HRad50 Protein Complex and Nijmegen Breakage Syndrome: Linkage of Double-Strand Break Repair to the Cellular DNA Damage Response. Cell 1998, 93, 477–486. [Google Scholar] [CrossRef]
- Centurione, L.; Aiello, F.B. DNA Repair and Cytokines: TGF-β, IL-6, and Thrombopoietin as Different Biomarkers of Radioresistance. Front. Oncol. 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.; Saba, K.H.; Magnusson, L.; Nilsson, J.; Karlsson, J.; Nord, K.H.; Gisselsson, D. Inactivation of RB1, CDKN2A, and TP53 Have Distinct Effects on Genomic Stability at Side-by-Side Comparison in Karyotypically Normal Cells. Genes, Chromosom. Cancer 2023, 62, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kedde, M.; le Sage, C.; Duursma, A.; Zlotorynski, E.; van Leeuwen, B.; Nijkamp, W.; Beijersbergen, R.; Agami, R. Telomerase-Independent Regulation of ATR by Human Telomerase RNA. J. Biol. Chem. 2006, 281, 40503–40514. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.S.Y.; Pohorelic, B.; Yu, Y.; Lees-Miller, S.P.; Beattie, T.L. The Human Telomerase RNA Component, HTR, Activates the DNA-Dependent Protein Kinase to Phosphorylate Heterogeneous Nuclear Ribonucleoprotein A1. Nucleic Acids Res. 2009, 37, 6105–6115. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR Is a Key Modulator of Ageing and Age-Related Disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- He, L.; Lu, J.; Yue, Z. Autophagy in Ageing and Ageing-Associated Diseases. Acta Pharmacol. Sin. 2013, 34, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; WHO Press: Geneva, Switzerland, 2015; pp. 28–30. [Google Scholar]
- Curtin, R.B.; Lowrie, E.G.; DeOreo, P.B. Self-Reported Functional Status: An Important Predictor of Health Outcomes among End-Stage Renal Disease Patients. Adv. Ren. Replace. Ther. 1999, 6, 133–140. [Google Scholar] [CrossRef]
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T.L. The Relationship between Functional Status, Physical Fitness and Cognitive Performance in Physically Active Older Adults: A Pilot Study. PLoS ONE 2018, 13, e0194918. [Google Scholar] [CrossRef]
- Reuben, D.B.; Seeman, T.E.; Keeler, E.; Hayes, R.P.; Bowman, L.; Sewall, A.; Hirsch, S.H.; Wallace, R.B.; Guralnik, J.M. Refining the Categorization of Physical Functional Status: The Added Value of Combining Self-Reported and Performance-Based Measures. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Škarić-Jurić, T.; Smolej Narančić, N.; Smolić, Š. Health-Risk Behaviours in Objective and Subjective Health among Croatians Aged 50 and Older. Drus. Istraz. 2020, 29, 217–239. [Google Scholar] [CrossRef]
- Cislaghi, B.; Cislaghi, C. Self-Rated Health as a Valid Indicator for Health-Equity Analyses: Evidence from the Italian Health Interview Survey. BMC Public Health 2019, 19, 533. [Google Scholar] [CrossRef]
- Wuorela, M.; Lavonius, S.; Salminen, M.; Vahlberg, T.; Viitanen, M.; Viikari, L. Self-Rated Health and Objective Health Status as Predictors of All-Cause Mortality among Older People: A Prospective Study with a 5-, 10-, and 27-Year Follow-Up. BMC Geriatr. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A Simple Salting Out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single Nucleotide Polymorphism Genotyping Using Kompetitive Allele Specific PCR (KASP): Overview of the Technology and Its Application in Crop Improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Perinić Lewis, A.; Škarić-Jurić, T.; Despot Lučanin, J.; Smolić, Š. The Lasting Impact of War Experiences on Quality of Life in Long-Lived Retirement Homes Residents: The Birth Cohort 1906–1928. Ageing Soc. 2022, 42, 1–29. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows 21.0; International Business Machines Corporation: Armonk, NY, USA, 2012.
- Shen, Q.; Zhang, Z.; Yu, L.; Cao, L.; Zhou, D.; Kan, M.; Li, B.; Zhang, D.; He, L.; Liu, Y. Common Variants Near TERC Are Associated with Leukocyte Telomere Length in the Chinese Han Population. Eur. J. Hum. Genet. 2011, 19, 721–723. [Google Scholar] [CrossRef]
- Šetinc, M.; Celinšćak, Ž.; Bočkor, L.; Ćorić, T.; Kolarić, B.; Stojanović Marković, A.; Zajc Petranović, M.; Peričić Salihović, M.; Smolej Narančić, N.; Škarić-Jurić, T. Genetic Scores for Predicting Longevity in the Croatian Oldest-Old Population. PLoS ONE 2023, 18, e0279971. [Google Scholar] [CrossRef]
- Fortney, K.; Dobriban, E.; Garagnani, P.; Pirazzini, C.; Monti, D.; Mari, D.; Atzmon, G.; Barzilai, N.; Franceschi, C.; Owen, A.B.; et al. Genome-Wide Scan Informed by Age-Related Disease Identifies Loci for Exceptional Human Longevity. PLoS Genet. 2015, 11, e1005728. [Google Scholar] [CrossRef]
- Dato, S.; Soerensen, M.; De Rango, F.; Rose, G.; Christensen, K.; Christiansen, L.; Passarino, G. The Genetic Component of Human Longevity: New Insights from the Analysis of Pathway-based SNP-SNP Interactions. Aging Cell 2018, 17, e12755. [Google Scholar] [CrossRef]
- Škarić-Jurić, T.; Celinšćak, Ž.; Šetinc, M.; Bočkor, L.; Stojanović Marković, A.; Zajc Petranović, M.; Peričić Salihović, M.; Deelen, J.; Janićijević, B.; Smolej Narančić, N. So Different but Equal: 33 Longevity Genes’ Loci in the Roma and in the General Population of Croatia. Anthropologie 2023, 61, 1–28. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Collins, K. Telomerase: An RNP Enzyme Synthesizes DNA. Cold Spring Harb. Perspect. Biol. 2011, 3, a003558. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Liu, B.; Helmling, C.; Sušac, L.; Cheng, R.; Zhou, Z.H.; Feigon, J. Structures of Telomerase at Several Steps of Telomere Repeat Synthesis. Nature 2021, 593, 454–459. [Google Scholar] [CrossRef]
- Harley, C.B.; Vaziri, H.; Counter, C.M.; Allsopp, R.C. The Telomere Hypothesis of Cellular Aging. Exp. Gerontol. 1992, 27, 375–382. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Panossian, L.A.; Porter, V.R.; Valenzuela, H.F.; Zhu, X.; Reback, E.; Masterman, D.; Cummings, J.L.; Effros, R.B. Telomere Shortening in T Cells Correlates with Alzheimer’s Disease Status. Neurobiol. Aging 2003, 24, 77–84. [Google Scholar] [CrossRef]
- von Zglinicki, T.; Serra, V.; Lorenz, M.; Saretzki, G.; Lenzen-Groβimlighaus, R.; Geβner, R.; Risch, A.; Steinhagen-Thiessen, E. Short Telomeres in Patients with Vascular Dementia: An Indicator of Low Antioxidative Capacity and a Possible Risk Factor? Lab. Investig. 2000, 80, 1739–1747. [Google Scholar] [CrossRef]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of Telomere Length Across Human Tissues. Science 2020, 369, 1–22. [Google Scholar] [CrossRef]
- Codd, V.; Mangino, M.; van der Harst, P.; Braund, P.S.; Kaiser, M.; Beveridge, A.J.; Rafelt, S.; Moore, J.; Nelson, C.; Soranzo, N.; et al. Common Variants Near TERC Are Associated with Mean Telomere Length. Nat. Genet. 2010, 42, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Scarabino, D.; Broggio, E.; Gambina, G.; Pelliccia, F.; Corbo, R. Common Variants of Human TERT and TERC Genes and Susceptibility to Sporadic Alzheimers Disease. Exp. Gerontol. 2017, 88, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Scarabino, D.; Peconi, M.; Pelliccia, F.; Corbo, R.M. Analysis of the Association Between TERC and TERT Genetic Variation and Leukocyte Telomere Length and Human Lifespan—A Follow-Up Study. Genes 2019, 10, 82. [Google Scholar] [CrossRef]
- Cong, Y.-S.; Wright, W.E.; Shay, J.W. Human Telomerase and Its Regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Saraga, E.P.; Bouzourene, H.; Bosman, F.T.; Benhattar, J. Expression of Telomerase Genes Correlates with Telomerase Activity in Human Colorectal Carcinogenesis. J. Pathol. 2001, 193, 21–26. [Google Scholar] [CrossRef]
- Feng, J.; Funk, W.D.; Wang, S.-S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.-P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J.; et al. The RNA Component of Human Telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef]
- Yashima, K.; Maitra, A.; Rogers, B.B.; Timmons, C.F.; Rathi, A.; Pinar, H.; Wright, W.E.; Shay, J.W.; Gazdar, A.F. Expression of the RNA Component of Telomerase During Human Development and Differentiation. Cell Growth Differ. 1998, 9, 805–813. [Google Scholar]
- Heinzel, S.; Marchingo, J.M.; Horton, M.B.; Hodgkin, P.D. The Regulation of Lymphocyte Activation and Proliferation. Curr. Opin. Immunol. 2018, 51, 32–38. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The Essential Kinase ATR: Ensuring Faithful Duplication of a Challenging Genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Manche, L.; Xu, R.-M.; Krainer, A.R. HnRNP A1 Associates with Telomere Ends and Stimulates Telomerase Activity. RNA 2006, 12, 1116–1128. [Google Scholar] [CrossRef]
- Paull, T.T.; Gellert, M. The 3′ to 5′ Exonuclease Activity of Mre11 Facilitates Repair of DNA Double-Strand Breaks. Mol. Cell 1998, 1, 969–979. [Google Scholar] [CrossRef]
- Trujillo, K.M.; Yuan, S.-S.F.; Lee, E.Y.-H.P.; Sung, P. Nuclease Activities in a Complex of Human Recombination and DNA Repair Factors Rad50, Mre11, and P95. J. Biol. Chem. 1998, 273, 21447–21450. [Google Scholar] [CrossRef]
- Coquel, F.; Silva, M.-J.; Técher, H.; Zadorozhny, K.; Sharma, S.; Nieminuszczy, J.; Mettling, C.; Dardillac, E.; Barthe, A.; Schmitz, A.-L.; et al. SAMHD1 Acts at Stalled Replication Forks to Prevent Interferon Induction. Nature 2018, 557, 57–61. [Google Scholar] [CrossRef]
- de Jager, M.; van Noort, J.; van Gent, D.C.; Dekker, C.; Kanaar, R.; Wyman, C. Human Rad50/Mre11 Is a Flexible Complex That Can Tether DNA Ends. Mol. Cell 2001, 8, 1129–1135. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Jin, K.; Wen, Z.; Cao, W.; Wu, B.; Wen, R.; Tian, L.; Berry, G.J.; Goronzy, J.J.; et al. The DNA Repair Nuclease MRE11A Functions as a Mitochondrial Protector and Prevents T Cell Pyroptosis and Tissue Inflammation. Cell Metab. 2019, 30, 477–492. [Google Scholar] [CrossRef]
- Helgadottir, A.; Thorleifsson, G.; Manolescu, A.; Gretarsdottir, S.; Blondal, T.; Jonasdottir, A.; Jonasdottir, A.; Sigurdsson, A.; Baker, A.; Palsson, A.; et al. A Common Variant on Chromosome 9p21 Affects the Risk of Myocardial Infarction. Science 2007, 316, 1491–1493. [Google Scholar] [CrossRef]
- McPherson, R.; Pertsemlidis, A.; Kavaslar, N.; Stewart, A.; Roberts, R.; Cox, D.R.; Hinds, D.A.; Pennacchio, L.A.; Tybjaerg-Hansen, A.; Folsom, A.R.; et al. A Common Allele on Chromosome 9 Associated with Coronary Heart Disease. Science 2007, 316, 1488–1491. [Google Scholar] [CrossRef]
- Kong, Y.; Hsieh, C.-H.; Alonso, L.C. ANRIL: A LncRNA at the CDKN2A/B Locus with Roles in Cancer and Metabolic Disease. Front. Endocrinol. 2018, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Pasmant, E.; Laurendeau, I.; Héron, D.; Vidaud, M.; Vidaud, D.; Bièche, I. Characterization of a Germ-Line Deletion, Including the Entire INK4/ARF Locus, in a Melanoma-Neural System Tumor Family: Identification of ANRIL, an Antisense Noncoding RNA Whose Expression Coclusters with ARF. Cancer Res. 2007, 67, 3963–3969. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Peters, G. Regulation of the INK4b-ARF-INK4a Tumour Suppressor Locus: All for One or One for All. Nat. Rev. Mol. Cell Biol. Vol. 2006, 7, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Congrains, A.; Kamide, K.; Ohishi, M.; Rakugi, H. ANRIL: Molecular Mechanisms and Implications in Human Health. Int. J. Mol. Sci. 2013, 14, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Pinós, T.; Fuku, N.; Cámara, Y.; Arai, Y.; Abe, Y.; Rodríguez-Romo, G.; Garatachea, N.; Santos-Lozano, A.; Miro-Casas, E.; Ruiz-Meana, M.; et al. The Rs1333049 Polymorphism on Locus 9p21.3 and Extreme Longevity in Spanish and Japanese Cohorts. Age 2014, 36, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Pilling, L.C.; Atkins, J.L.; Bowman, K.; Jones, S.E.; Tyrrell, J.; Beaumont, R.N.; Ruth, K.S.; Tuke, M.A.; Yaghootkar, H.; Wood, A.R.; et al. Human Longevity Is Influenced by Many Genetic Variants: Evidence from 75,000 UK Biobank Participants. Aging 2016, 8, 547–560. [Google Scholar] [CrossRef]
- Timmers, P.R.H.J.; Wilson, J.F.; Joshi, P.K.; Deelen, J. Multivariate Genomic Scan Implicates Novel Loci and Haem Metabolism in Human Ageing. Nat. Commun. 2020, 11, 3570. [Google Scholar] [CrossRef]
- Pellatt, A.J.; Wolff, R.K.; John, E.M.; Torres-Mejia, G.; Hines, L.M.; Baumgartner, K.B.; Giuliano, A.R.; Lundgreen, A.; Slattery, M.L. SEPP1 Influences Breast Cancer Risk among Women with Greater Native American Ancestry: The Breast Cancer Health Disparities Study. PLoS ONE 2013, 8, e80554. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From Cytokine to Myokine: The Emerging Role of Interleukin-6 in Metabolic Regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a Disease of the Innate Immune System: Association of Acute-Phase Reactants and Interleukin-6 with Metabolic Syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, D.; Vasto, S.; Capurso, C.; Christiansen, L.; Deiana, L.; Franceschi, C.; Hurme, M.; Mocchegiani, E.; Rea, M.; Lio, D.; et al. Effect of Interleukin-6 Polymorphisms on Human Longevity: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2009, 8, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kayaaltı, Z.; Şahiner, L.; Durakoğlugil, M.E.; Söylemezoğlu, T. Distributions of Interleukin-6 (IL-6) Promoter and Metallothionein 2A (MT2A) Core Promoter Region Gene Polymorphisms and Their Associations with Aging in Turkish Population. Arch. Gerontol. Geriatr. 2011, 53, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Persson, M.; Andersson, P.; Stenvinkel, P.; Nordfors, L.; Madden, J.; Vedin, I.; Wretlind, B.; Grimble, R.F.; Palmblad, J. Polymorphisms in Cytokine Genes Influence Long-Term Survival Differently in Elderly Male and Female Patients. J. Intern. Med. 2007, 262, 215–223. [Google Scholar] [CrossRef]
- Capurso, C.; Solfrizzi, V.; D’Introno, A.; Colacicco, A.M.; Capurso, S.A.; Capurso, A.; Panza, F. Interleukin 6-174 G/C Promoter Gene Polymorphism and Sporadic Alzheimer’s Disease: Geographic Allele and Genotype Variations in Europe. Exp. Gerontol. 2004, 39, 1567–1573. [Google Scholar] [CrossRef]
- Wang, B.; Cai, Z.; Tao, K.; Zeng, W.; Lu, F.; Yang, R.; Feng, D.; Gao, G.; Yang, Q. Essential Control of Mitochondrial Morphology and Function by Chaperone-Mediated Autophagy through Degradation of PARK7. Autophagy 2016, 12, 1215–1228. [Google Scholar] [CrossRef]
- Niki, T.; Takahashi-Niki, K.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. DJBP: A Novel DJ-1-Binding Protein, Negatively Regulates the Androgen Receptor by Recruiting Histone Deacetylase Complex, and DJ-1 Antagonizes This Inhibition by Abrogation of This Complex. Mol. Cancer Res. 2003, 1, 247–261. [Google Scholar]
- Flachsbart, F.; Ellinghaus, D.; Gentschew, L.; Heinsen, F.-A.; Caliebe, A.; Christiansen, L.; Nygaard, M.; Christensen, K.; Blanché, H.; Deleuze, J.-F.; et al. Immunochip Analysis Identifies Association of the RAD50/IL13 Region with Human Longevity. Aging Cell 2016, 15, 585–588. [Google Scholar] [CrossRef]
- Vu, T.T.; Lu, W.; Weiss, M.; Nguyen, L.T.; Ngo, V.K. Mental Health, Functional Impairment, and Barriers to Mental Health Access among Cancer Patients in Vietnam. Psychooncology 2023, 32, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Muhandiramge, J.; Orchard, S.G.; Warner, E.T.; van Londen, G.J.; Zalcberg, J.R. Functional Decline in the Cancer Patient: A Review. Cancers 2022, 14, 1368. [Google Scholar] [CrossRef]
- Castellanos-Perilla, N.; Borda, M.G.; Fernández-Quilez, Á.; Aarsland, V.; Soennesyn, H.; Cano-Gutiérrez, C.A. Factors Associated with Functional Loss among Community-Dwelling Mexican Older Adults. Biomédica 2020, 40, 546–556. [Google Scholar] [CrossRef]
- Mutz, J.; Lewis, C.M. Cross-Classification between Self-Rated Health and Health Status: Longitudinal Analyses of All-Cause Mortality and Leading Causes of Death in the UK. Sci. Rep. 2022, 12, 459. [Google Scholar] [CrossRef]
- Latham, K.; Peek, C.W. Self-Rated Health and Morbidity Onset among Late Midlife U.S. Adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2013, 68, 107–116. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kobayashi, E.; Fukaya, T.; Takahashi, Y.; Shinkai, S.; Liang, J. Association of Physical Performance and Self-Rated Health with Multimorbidity among Older Adults: Results from a Ntionwide Survey in Japan. Arch. Gerontol. Geriatr. 2019, 84, 103904. [Google Scholar] [CrossRef] [PubMed]
| SNP | Closest Gene | Chromosome Position (GRCh38) | Alleles (Major/Minor) | MAF | Genotyping Success Rate | HWE p-Value with Yates’s Correction * |
|---|---|---|---|---|---|---|
| rs225119 | PARK7 | 1:7984301 | C/T | 0.425 | 0.979 | 0.815 |
| rs16847897 | TERC | 3:169850328 | G/C | 0.292 | 0.979 | 0.996 |
| rs2706372 | RAD50/IL13 region | 5:132599785 | C/T | 0.272 | 0.960 | 0.997 |
| rs1800795 | IL6 | 7:22727026 | G/C | 0.448 | 0.948 | 0.562 |
| rs4977756 | CDKN2B/ANRIL | 9:22068653 | A/G | 0.399 | 0.966 | 0.959 |
| rs1333049 | CDKN2A | 9:22125504 | G/C | 0.470 | 0.972 | 0.319 |
| rs533984 | MRE11A | 11:94466106 | G/A | 0.396 | 0.960 | 0.589 |
| rs17202060 | TXNRD1 | 12:104337068 | C/T | 0.336 | 0.960 | 0.812 |
| rs1042522 | TP53 | 17:7676154 | C/G | 0.238 | 0.976 | 0.448 |
| rs50871 | ERCC2 | 19:45359257 | A/C | 0.460 | 0.966 | 0.902 |
| Gene (rsID) | p-Value ANOVA | Variables | Means in More Frequent Homozygotes | Means in Heterozygotes | Means in Less Frequent Homozygotes | Genotypes with Significant Differences | p-Value Post Hoc Test |
|---|---|---|---|---|---|---|---|
| TERC (rs16847897) | 0.001 | Sum: self-rated health AND mobility AND independence | 9.18 | 8.02 | 7.44 | GG:CC GG:GC | 0.005 0.001 |
| 0.005 | Sum: self-rated mobility AND independence | 6.35 | 5.59 | 5.30 | GG:CC GG:GC | 0.022 0.005 | |
| 0.005 | Sum: self-rated health AND self-rated health compared to that of their age peers AND mobility AND independence | 11.81 | 10.63 | 10.08 | GG:CC GG:GC | 0.018 0.006 | |
| 0.006 | Sum: self-rated health AND self-rated health compared to that of their age peers | 5.48 | 4.97 | 4.72 | GG:CC GG:GC | 0.019 0.007 | |
| 0.014 | MMSE | 23.34 | 21.61 | 23.74 | GC:CC GC:GG | 0.060 0.007 | |
| MRE11A (rs533984) | 0.005 | Sum: self-rated mobility AND independence | 6.04 | 5.60 | 6.84 | AA:GA AA:GG | 0.001 0.044 |
| 0.016 | Sum: self-rated health AND mobility AND independence | 8.52 | 8.21 | 9.72 | AA:GA AA:GG | 0.004 0.028 | |
| 0.029 | Sum: self-rated health AND self-rated health compared to that of their age peers AND mobility AND independence | 11.13 | 10.82 | 12.45 | AA:GA AA:GG | 0.008 0.038 | |
| CDKN2B (rs4977756) | 0.032 | Sum: self-rated health AND self-rated health compared to that of their age peers AND mobility AND independence | 10.60 | 11.28 | 12.22 | GG:AA | 0.010 |
| 0.046 | Sum: self-rated health AND mobility AND independence | 8.11 | 8.58 | 9.43 | GG:AA | 0.014 | |
| TXNRD1 (rs17202060) | 0.024 | Number of drugs per day | 2.14 | 2.24 | 1.79 | TT:CC TT:TC | 0.033 0.006 |
| 0.038 | Number of diseases | 6.14 | 5.49 | 5.24 | CC:TC CC:TT | 0.030 0.046 | |
| IL6 (rs1800795) | 0.028 | Number of drugs per day | 1.95 | 2.21 | 2.28 | GG:CC GG:GC | 0.018 0.025 |
| PARK7 (rs225119) | 0.030 | Number of diseases | 5.65 | 5.53 | 6.54 | TT:CC TT:TC | 0.033 0.009 |
| Gene (rsID) | Variables | Class Where the Genotype(s) Is More Frequent | More Frequent Genotype(s) | Minor Allele | p-Value |
|---|---|---|---|---|---|
| TERC (rs16847897) | Sum: self-rated mobility AND independence | 7–10 | GG | C | 0.00026 |
| Self-rated health | good, very good, excellent | GG | C | 0.00133 | |
| Sum: self-rated health AND mobility AND independence | 7–15 | GG | C | 0.00148 | |
| Sum: self-rated health AND self-rated health compared to that of their age peers | 6–8 | GG | C | 0.004 | |
| Self-rated mobility | good, very good, excellent | GG | C | 0.005 | |
| Sum: self-rated health AND self-rated health compared to that of their age peers AND mobility AND independence | 10–18 | GG | C | 0.021 | |
| Self-rated independence | good, very good, excellent | GG | C | 0.029 | |
| MMSE score | 18+ | GG, CC | C | 0.010 | |
| MRE11A (rs533984) | Sum: self-rated mobility AND independence | 7–10 | AA | A | 0.006 |
| Self-rated independence | very good, excellent | AA, GG | A | 0.009 | |
| Number of drugs per day/by median/ | 0–2 drugs per day | AA | A | 0.005 | |
| CDKN2B (rs4977756) | Sum: self-rated health AND self-rated health compared to that of their age peers | 6–8 | GG | G | 0.013 |
| Self-rated mobility | very good, excellent | GG | G | 0.018 | |
| Self-rated independence | very good, excellent | GG, GA | G | 0.039 | |
| CDKN2A (rs1333049) | Self-rated mobility | very good, excellent | GG | C | 0.031 |
| PARK7 (rs225119) | Number of diseases | Men: 6–12; Women: 7–12 | TT | T | 0.034 |
| RAD50 (rs2706372) | Number of diseases | Men: 6–12; Women: 7–12 | CC, TT | T | 0.026 |
| TXNRD1 (rs17202060) | Number of drugs per day | 4+ (in both genders) | TC | T | 0.050 |
| Variables | p-Value | OR | 95%CI |
|---|---|---|---|
| Gender (women are referent) | 0.399 | 1.319 | 0.694–2.507 |
| Age at measurement (years) | 0.024 | 0.974 | 0.951–0.996 |
| MMSE score | 0.001 | 1.125 | 1.053–1.203 |
| Number of chronic diseases and conditions | 0.000 | 0.738 | 0.645–0.844 |
| Number of drugs per day | 0.682 | 0.974 | 0.859–1.105 |
| TERC (rs16847897) | 0.001 | ||
| CC, GG are referent (vs. GC) | 0.851 | 0.900 | 0.300–2.702 |
| CC, GC are referent (vs. GG) | 0.076 | 2.648 | 0.903–7.763 |
| MRE11A (rs533984) | 0.002 | ||
| GA, GG are referent (vs. AA) | 0.037 | 2.536 | 1.055–6.094 |
| AA, GG are referent (vs. GA) | 0.066 | 0.566 | 0.309–1.038 |
| CDKN2B (rs4977756) | 0.063 | ||
| AA, GG are referent (vs. GA) | 0.994 | 1.002 | 0.502–2.003 |
| AA, GA are referent (vs. GG) | 0.040 | 2.966 | 1.051–8.369 |
| CDKN2A (rs1333049) | 0.439 | 1.051–8.369 | |
| GC, GG are referent (vs. CC) | 0.543 | 1.340 | 0.522–3.445 |
| CC, GG are referent (vs. GC) | 0.207 | 1.640 | 0.761–3.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šetinc, M.; Zajc Petranović, M.; Slivšek, G.; Mijač, S.; Celinščak, Ž.; Stojanović Marković, A.; Bišof, V.; Peričić Salihović, M.; Škarić-Jurić, T. Genes Involved in DNA Damage Cell Pathways and Health of the Oldest-Old (85+). Genes 2023, 14, 1806. https://doi.org/10.3390/genes14091806
Šetinc M, Zajc Petranović M, Slivšek G, Mijač S, Celinščak Ž, Stojanović Marković A, Bišof V, Peričić Salihović M, Škarić-Jurić T. Genes Involved in DNA Damage Cell Pathways and Health of the Oldest-Old (85+). Genes. 2023; 14(9):1806. https://doi.org/10.3390/genes14091806
Chicago/Turabian StyleŠetinc, Maja, Matea Zajc Petranović, Goran Slivšek, Sandra Mijač, Željka Celinščak, Anita Stojanović Marković, Vesna Bišof, Marijana Peričić Salihović, and Tatjana Škarić-Jurić. 2023. "Genes Involved in DNA Damage Cell Pathways and Health of the Oldest-Old (85+)" Genes 14, no. 9: 1806. https://doi.org/10.3390/genes14091806