Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy
Abstract
:1. Introduction
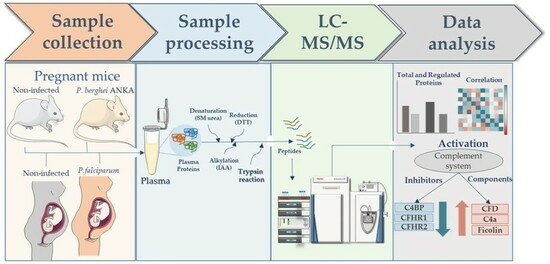
2. Materials and Methods
2.1. Mice and Parasites Strains
2.2. Bodyweight and Parasitemia Measurement during Infection
2.3. Mouse Cytokine Measurements Using Cytometric Bead Array
2.4. Birth Parameters, Vascular Space, and Spleen Weight Measurements
2.5. Study Design of Human Malaria in a Pregnancy Cohort
2.6. Blood Sample Collection and Plasma Isolation
2.7. Plasma Protein Extraction and Digestion for Mass Spectrometry
2.8. Mass Spectrometry Analysis
2.9. Database Search and Statistical Analysis
2.10. CBA Anaphylatoxin Assay
2.11. Proteomics and Clinical Parameters Correlation
3. Results
3.1. Establishment of a Murine Translational Model of Malaria in Pregnancy for Biomarker Discovery
3.2. Complement System Activation in Malaria in the Pregnancy Context
3.3. Anaphylatoxin Measurement Confirms the Activation of the Complement System
3.4. Proteomics and Clinical Data Correlation in MiP
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fried, M.; Duffy, P.E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996, 272, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Ayres Pereira, M.; Mandel Clausen, T.; Pehrson, C.; Mao, Y.; Resende, M.; Daugaard, M.; Riis Kristensen, A.; Spliid, C.; Mathiesen, L.; Knudsen, L.E. Placental sequestration of Plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate A on syndecan-1. PLoS Pathog. 2016, 12, e1005831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogerson, S.J.; Hviid, L.; Duffy, P.E.; Leke, R.F.G.; Taylor, D.W. Malaria in pregnancy: Pathogenesis and immunity. Lancet Infect. Dis. 2007, 7, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Moore, K.A.; Baiwog, F.; Ura, A.; Clapham, C.; King, C.L.; Siba, P.M.; Beeson, J.G.; Mueller, I.; Fowkes, F.J. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 313–324. [Google Scholar] [CrossRef]
- Suguitan Jr, A.L.; Leke, R.G.F.; Fouda, G.; Zhou, A.; Thuita, L.; Metenou, S.; Fogako, J.; Megnekou, R.; Taylor, D.W. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 2003, 188, 1074–1082. [Google Scholar] [CrossRef] [Green Version]
- Umbers, A.J.; Aitken, E.H.; Rogerson, S.J. Malaria in pregnancy: Small babies, big problem. Trends Parasitol. 2011, 27, 168–175. [Google Scholar] [CrossRef]
- Lambris, J.D.; Ricklin, D.; Geisbrecht, B.V. Complement evasion by human pathogens. Nat. Rev. Microbiol. 2008, 6, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- Albieri, A.; Kipnis, T.; Bevilacqua, E. A possible role for activated complement component 3 in phagocytic activity exhibited by the mouse trophoblast. Am. J. Reprod. Immunol. 1999, 41, 343–352. [Google Scholar] [CrossRef]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Wirstlein, P.K.; Jasiński, P.; Rajewski, M.; Goździewicz, T.; Skrzypczak, J. Complement inhibitory proteins expression in placentas of thrombophilic women. Folia Histochem. Cytobiol. 2012, 50, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, M.; McCall, M.; Mollnes, T.E.; van Deuren, M.; Sprong, T.; Klasen, I.; Hermsen, C.C.; Sauerwein, R.W.; van der Ven, A. Complement activation in experimental human malaria infection. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Nyakoe, N.K.; Taylor, R.P.; Makumi, J.N.; Waitumbi, J.N. Complement consumption in children with Plasmodium falciparum malaria. Malar. J. 2009, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, K.L.; Higgins, S.J.; McDonald, C.R.; Kain, K.C. Complement driven innate immune response to malaria: Fuelling severe malarial diseases. Cell Microbiol. 2010, 12, 1036–1045. [Google Scholar] [CrossRef]
- Mibei, E.K.; Orago, A.S.; Stoute, J.A. Immune complex levels in children with severe Plasmodium falciparum malaria. Am. J. Trop Med. Hyg. 2005, 72, 593–599. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Yin, W.; Grigg, S.E.; Ghebrehiwet, B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 2006, 4, 2035–2042. [Google Scholar] [CrossRef]
- Conroy, A.; Serghides, L.; Finney, C.; Owino, S.O.; Kumar, S.; Gowda, D.C.; Liles, W.C.; Moore, J.M.; Kain, K.C. C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: Potential implications for placental malaria. PLoS ONE 2009, 4, e4953. [Google Scholar] [CrossRef] [Green Version]
- Vaisbuch, E.; Romero, R.; Erez, O.; Mazaki-Tovi, S.; Kusanovic, J.P.; Soto, E.; Gotsch, F.; Dong, Z.; Chaiworapongsa, T.; Kim, S.K.; et al. Fragment Bb in amniotic fluid: Evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J. Matern. Fetal Neonatal Med. 2009, 22, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Soto, E.; Romero, R.; Richani, K.; Espinoza, J.; Nien, J.K.; Chaiworapongsa, T.; Santolaya-Forgas, J.; Edwin, S.S.; Mazor, M. Anaphylatoxins in preterm and term labor. J. Perinat. Med. 2005, 33, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Conroy, A.L.; Silver, K.L.; Zhong, K.; Rennie, M.; Ward, P.; Sarma, J.V.; Molyneux, M.E.; Sled, J.; Fletcher, J.F.; Rogerson, S. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe 2013, 13, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Silver, K.L.; Conroy, A.L.; Leke, R.G.; Leke, R.J.; Gwanmesia, P.; Molyneux, M.E.; Taylor, D.W.; Rogerson, S.J.; Kain, K.C. Circulating soluble endoglin levels in pregnant women in Cameroon and Malawi--associations with placental malaria and fetal growth restriction. PLoS ONE 2011, 6, e24985. [Google Scholar] [CrossRef]
- Ataíde, R.; Murillo, O.; Dombrowski, J.G.; Souza, R.M.; Lima, F.A.; Lima, G.F.; Hristov, A.D.; Valle, S.C.; Di Santi, S.M.; Epiphanio, S.; et al. Malaria in Pregnancy Interacts with and Alters the Angiogenic Profiles of the Placenta. PLoS Negl. Trop. Dis. 2015, 9, e0003824. [Google Scholar] [CrossRef]
- Lufele, E.; Umbers, A.; Ordi, J.; Ome-Kaius, M.; Wangnapi, R.; Unger, H.; Tarongka, N.; Siba, P.; Mueller, I.; Robinson, L.; et al. Risk factors and pregnancy outcomes associated with placental malaria in a prospective cohort of Papua New Guinean women. Malar. J. 2017, 16, 427. [Google Scholar] [CrossRef] [Green Version]
- Gueneuc, A.; Deloron, P.; Bertin, G.I. Usefulness of a biomarker to identify placental dysfunction in the context of malaria. Malar. J. 2017, 16, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, R.; Rosa-Fernandes, L.; Dos Santos, A.F.; Bandeira, C.L.; Dombrowski, J.G.; Souza, R.M.; Da Fonseca, M.P.; Festuccia, W.T.; Labriola, L.; Larsen, M.R.; et al. Integrated Proteomics Reveals Apoptosis-related Mechanisms Associated with Placental Malaria. Mol. Cell. Proteom. 2019, 18, 182–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neres, R.; Marinho, C.R.; Gonçalves, L.A.; Catarino, M.B.; Penha-Gonçalves, C. Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS ONE 2008, 3, e1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, C.R.F.; Neres, R.; Epiphanio, S.; Gonçalves, L.A.; Catarino, M.B.; Penha-Gonçalves, C. Recrudescent Plasmodium berghei from Pregnant Mice Displays Enhanced Binding to the Placenta and Induces Protection in Multigravida. PLoS ONE 2009, 4, e5630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombrowski, J.G.; Barateiro, A.; Peixoto, E.P.M.; Barros, A.; Souza, R.M.; Clark, T.G.; Campino, S.; Wrenger, C.; Wunderlich, G.; Palmisano, G.; et al. Adverse pregnancy outcomes are associated with Plasmodium vivax malaria in a prospective cohort of women from the Brazilian Amazon. PLoS Negl. Trop. Dis. 2021, 15, e0009390. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Beattie, L.; Amante, F.H. The importance of the spleen in malaria. Trends Parasitol. 2005, 21, 75–80. [Google Scholar] [CrossRef]
- Brabin, B.J.; Romagosa, C.; Abdelgalil, S.; Menéndez, C.; Verhoeff, F.H.; McGready, R.; Fletcher, K.A.; Owens, S.; D’Alessandro, U.; Nosten, F.; et al. The sick placenta-the role of malaria. Placenta 2004, 25, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.T.; Brown, H.; Chensue, S.W.; Turner, G.D.; Tadesse, E.; Lema, V.M.; Molyneux, M.E.; Rochford, R.; Meshnick, S.R.; Rogerson, S.J. Host response to malaria during pregnancy: Placental monocyte recruitment is associated with elevated β chemokine expression. J. Immunol. 2003, 170, 2759–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGready, R.; Brockman, A.; Cho, T.; Levesque, M.A.; Tkachuk, A.N.; Meshnick, S.R.; Nosten, F. Haemozoin as a marker of placental parasitization. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.L.; Funk, S.G.; Frothingham, T.E. Disproportionate intra-uterine head growth and developmental outcome. Dev. Med. Child. Neurol. 1985, 27, 746–750. [Google Scholar] [CrossRef]
- Passemard, S.; Kaindl, A.M.; Verloes, A. Chapter 13—Microcephaly. In Handbook of Clinical Neurology; Dulac, O., Lassonde, M., Sarnat, H.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 111, pp. 129–141. [Google Scholar]
- World Health Organization; Nutrition for Health. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Jacobs, P.; Radzioch, D.; Stevenson, M.M. In vivo regulation of nitric oxide production by tumor necrosis factor α and γ interferon, but not by interleukin-4, during blood stage malaria in mice. Infect. Immun. 1996, 64, 44–49. [Google Scholar] [CrossRef]
- Kabyemela, E.R.; Fried, M.; Kurtis, J.D.; Mutabingwa, T.K.; Duffy, P.E. Fetal responses during placental malaria modify the risk of low birth weight. Infect. Immun. 2008, 76, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Alkadarou, T.; Musa, A.; Alkadarou, A.; Mahfouz, M.S.; Troye-Blomberg, M.; Elhassan, A.M.; Elhassan, I.M. Immunological Characteristics of Hyperreactive Malarial Splenomegaly Syndrome in Sudanese Patients. J. Trop. Med. 2013, 2013, 961051. [Google Scholar] [CrossRef] [Green Version]
- Gosi, P.; Khusmith, S.; Looareesuwan, S.; Sitachamroom, U.; Glanarongran, R.; Buchachart, K.; Walsh, D.S. Complicated malaria is associated with differential elevations in serum levels of interleukins 10, 12, and 15. Southeast Asian J. Trop. Med. Public. Health 1999, 30, 412–417. [Google Scholar] [PubMed]
- Hugosson, E.; Montgomery, S.M.; Premji, Z.; Troye-Blomberg, M.; Björkman, A. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol. 2004, 26, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Dombrowski, J.G.; Souza, R.M.; Lima, F.A.; Bandeira, C.L.; Murillo, O.; Costa, D.S.; Peixoto, E.P.M.; Cunha, M.D.P.; Zanotto, P.M.A.; Bevilacqua, E.; et al. Association of Malaria Infection During Pregnancy With Head Circumference of Newborns in the Brazilian Amazon. JAMA Netw. Open. 2019, 2, e193300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Beek, A.E.; Sarr, I.; Correa, S.; Nwakanma, D.; Brouwer, M.C.; Wouters, D.; Secka, F.; Anderson, S.T.B.; Conway, D.J.; Walther, M.; et al. Complement Factor H Levels Associate With Plasmodium falciparum Malaria Susceptibility and Severity. Open Forum Infect. Dis. 2018, 5, ofy166. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D.; Smith, G.M.; Poovassery, J.S.; Nagy, T.; Moore, J.M. Plasmodium chabaudi AS induces pregnancy loss in association with systemic pro-inflammatory immune responses in A/J and C57BL/6 mice. Parasite Immunol. 2012, 34, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Poovassery, J.; Moore, J.M. Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta. Infect. Immun. 2006, 74, 2839–2848. [Google Scholar] [CrossRef] [Green Version]
- Bulmer, J.N.; Rasheed, F.N.; Francis, N.; Morrison, L.; Greenwood, B.M. Placental malaria. I. Pathological classification. Histopathology 1993, 22, 211–218. [Google Scholar] [CrossRef]
- Ozawa, K.; Hashimoto, K.; Kishimoto, T.; Shimizu, E.; Ishikura, H.; Iyo, M. Immune Activation During Pregnancy in Mice Leads to Dopaminergic Hyperfunction and Cognitive Impairment in the Offspring: A Neurodevelopmental Animal Model of Schizophrenia. Biol. Psychiatry 2006, 59, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [Green Version]
- Richani, K.; Soto, E.; Romero, R.; Espinoza, J.; Chaiworapongsa, T.; Nien, J.K.; Edwin, S.; Kim, Y.M.; Hong, J.S.; Mazor, M. Normal pregnancy is characterized by systemic activation of the complement system. J. Matern. Fetal Neonatal Med. 2005, 17, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Derzsy, Z.; Prohászka, Z.; Rigó, J., Jr.; Füst, G.; Molvarec, A. Activation of the complement system in normal pregnancy and preeclampsia. Mol. Immunol. 2010, 47, 1500–1506. [Google Scholar] [CrossRef]
- Conroy, A.L.; McDonald, C.R.; Silver, K.L.; Liles, W.C.; Kain, K.C. Complement activation: A critical mediator of adverse fetal outcomes in placental malaria? Trends Parasitol. 2011, 27, 294–299. [Google Scholar] [CrossRef]
- McDonald, C.R.; Elphinstone, R.E.; Kain, K.C. The impact of placental malaria on neurodevelopment of exposed infants: A role for the complement system? Trends Parasitol. 2013, 29, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; Kremsner, P.G.; Meri, S. Complement activation in primiparous women from a malaria endemic area is associated with reduced birthweight. Placenta 2013, 34, 162–167. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifati, D.M.; Kishore, U. Role of complement in neurodegeneration and neuroinflammation. Mol. Immunol. 2007, 44, 999–1010. [Google Scholar] [CrossRef] [PubMed]








| Protein ID | Protein Name | Placental Vascular Space | Fetus Weight | Spleen Weight | IL-10 |
|---|---|---|---|---|---|
| P98086 | Complement C1q subcomponent subunit A | −0.76376 | −0.76376 | 0.78571 | |
| P14106 | Complement C1q subcomponent subunit B | −0.87287 | −0.87287 | 0.92857 | 0.74253 |
| Q02105 | Complement C1q subcomponent subunit C | 0.78571 | |||
| Q8K182 | Complement component C8 alpha chain | −0.76376 | −0.76376 | 0.78571 | 0.74253 |
| Q8VCG4 | Complement component C8 gamma chain | −0.87287 | −0.87287 | 0.7619 | 0.81439 |
| P11680 | Properdin | −0.87287 | 0.92857 | 0.77846 |
| Protein ID | Protein Name | Placental Malaria | Newborn Weight | Newborn Head Circumference | Newborn Chest |
|---|---|---|---|---|---|
| P20851 | C4b-binding protein beta chain | −0.5906 | 0.72381 | ||
| Q9NZP8 | Complement C1r subcomponent-like protein | −0.47703 | 0.50891 | 0.60161 | |
| Q15485 | Ficolin-2 | −0.59848 | |||
| P36980 | Complement Factor H-related protein 2 | 0.6225 | 0.48617 | ||
| P0C0L4 | Complement C4-A | −0.64629 | −0.47836 | −0.58152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, V.F.; Dombrowski, J.G.; Kawahara, R.; Rosa-Fernandes, L.; Mule, S.N.; Murillo, O.; Santana, T.V.; Coutinho, J.V.P.; Macedo-da-Silva, J.; Lazari, L.C.; et al. Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy. Genes 2023, 14, 1624. https://doi.org/10.3390/genes14081624
Santiago VF, Dombrowski JG, Kawahara R, Rosa-Fernandes L, Mule SN, Murillo O, Santana TV, Coutinho JVP, Macedo-da-Silva J, Lazari LC, et al. Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy. Genes. 2023; 14(8):1624. https://doi.org/10.3390/genes14081624
Chicago/Turabian StyleSantiago, Veronica Feijoli, Jamille Gregorio Dombrowski, Rebeca Kawahara, Livia Rosa-Fernandes, Simon Ngao Mule, Oscar Murillo, Thais Viggiani Santana, Joao Victor Paccini Coutinho, Janaina Macedo-da-Silva, Lucas Cardoso Lazari, and et al. 2023. "Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy" Genes 14, no. 8: 1624. https://doi.org/10.3390/genes14081624