Association between the rs820218 Variant within the SAP30BP Gene and Rotator Cuff Rupture in an Amazonian Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. DNA Collection
2.3. Single-Nucleotide Polymorphism Selection and Genotyping
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longo, U.G.; Margiotti, K.; Petrillo, S.; Rizzello, G.; Fusilli, C.; Maffulli, N.; De Luca, A.; Denaro, V. Genetic of rotator cuff tears: No association of col5al gene in a case-control study. BMC Med. Genet. 2018, 19, 217. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Shin, D.C.; Kim, K. Prevalence of asymptomatic rotator cuff tear and their related factors in the Korean population. J. Shoulder Elb. Surg. 2017, 26, 30–35. [Google Scholar] [CrossRef]
- Roos, T.R.; Roos, A.K.; Avins, A.L.; Ahmed, M.A.; Kleimeyer, J.P.; Fredericson, M.; Ioannidis, J.P.A.; Dragoo, J.L.; Kim, S.K. Genome-wide association study identifies a locus associated with rotator cuff injury. PLoS ONE 2017, 12, e0189317. [Google Scholar] [CrossRef] [Green Version]
- Tashjian, R.Z. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin. Sports Med. 2012, 31, 589–604. [Google Scholar] [CrossRef]
- Yamamoto, A.; Takagishi, K.; Osawa, T.; Yanagawa, T.; Nakajima, D.; Shitara, H.; Kobayashi, T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elb. Surg. 2010, 19, 116–120. [Google Scholar] [CrossRef]
- Jain, N.B.; Luz, J.; Higgins, L.D.; Dong, Y.; Warner, J.J.; Matzkin, E.; Katz, J.N. The Diagnostic Accuracy of Special Tests for Rotator Cuff Tear: The ROW Cohort Study. Am. J. Phys. Med. Rehabil. 2017, 96, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Tashjian, R.Z.; Kim, S.K.; Roche, M.D.; Jones, K.B.; Teerlink, C.C. Genetic variants associated with rotator cuff tearing utilizing multiple population-based genetic resources. J. Shoulder Elb. Surg. 2021, 30, 520–531. [Google Scholar] [CrossRef]
- Assunção, J.H.; Godoy-Santos, A.L.; Dos Santos, M.C.L.G.; Malavolta, E.A.; Gracitelli, M.E.C.; Ferreira Neto, A.A. Matrix Metalloproteases 1 and 3 Promoter Gene Polymorphism Is Associated With Rotator Cuff Tear. Clin. Orthop. Relat. Res. 2017, 475, 1904–1910. [Google Scholar] [CrossRef] [Green Version]
- Gumina, S.; Villani, C.; Arceri, V.; Fagnani, C.; Nisticò, L.; Venditto, T.; Castagna, A.; Candela, V. Rotator Cuff Degeneration: The Role of Genetics. J. Bone Joint Surg. Am. 2019, 101, 600–605. [Google Scholar] [CrossRef]
- Longo, U.G.; Candela, V.; Berton, A.; Salvatore, G.; Guarnieri, A.; DeAngelis, J.; Nazarian, A.; Denaro, V. Genetic basis of rotator cuff injury: A systematic review. BMC Med. Genet. 2019, 20, 149. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Farnham, J.M.; Albright, F.S.; Teerlink, C.C.; Cannon-Albright, L.A. Evidence for an Inherited Predisposition Contributing to the Risk for Rotator Cuff Disease. JBJS 2009, 91, 1136. [Google Scholar] [CrossRef] [Green Version]
- da Rocha Motta, G.; Amaral, M.V.; Rezende, E.; Pitta, R.; dos Santos Vieira, T.C.; Duarte, M.E.L.; Vieira, A.R.; Casado, P.L. Evidence of genetic variations associated with rotator cuff disease. J. Shoulder Elb. Surg. 2014, 1, 227–235. [Google Scholar] [CrossRef]
- Kluger, R.; Burgstaller, J.; Vogl, C.; Brem, G.; Skultety, M.; Mueller, S. Candidate gene approach identifies six SNPs in tenascin-C (TNC) associated with degenerative rotator cuff tears. J. Orthop. Res. 2017, 35, 894–901. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.S.; Ok, J.H.; Song, H.J. Apoptosis occurs throughout the diseased rotator cuff. Am. J. Sports Med. 2013, 41, 2249–2255. [Google Scholar] [CrossRef]
- Lundgreen, K.; Lian, Ø.; Scott, A.; Engebretsen, L. Increased levels of apoptosis and p53 in partial-thickness supraspinatus tendon tears. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1636–1641. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Granger, E.K.; Farnham, J.M.; Cannon-Albright, L.A.; Teerlink, C.C. Genome-wide association study for rotator cuff tears identifies two significant single-nucleotide polymorphisms. J. Shoulder Elb. Surg. 2016, 25, 174–179. [Google Scholar] [CrossRef]
- Tian, B.; Kang, X.; Zhang, L.; Zheng, J.; Zhao, Z. SAP30BP gene is associated with the susceptibility of rotator cuff tear: A case-control study based on Han Chinese population. J. Orthop. Surg. Res. 2020, 15, 356. [Google Scholar] [CrossRef]
- Li, J.-F.; Liu, L.-D.; Ma, S.-H.; Cheng-Hong, D.; Wang, L.-C.; Dong, C.-H.; Zhao, H.-L.; Liao, Y.; Li, Q.-H. HTRP—An Immediate-Early Gene Product Induced by HSV1 Infection in Human Embryo Fibroblasts, Is Involved in Cellular Co-Repressors. J. Biochem. 2004, 136, 169–176. [Google Scholar] [CrossRef]
- Küchler, E.C.; Tannure, P.N.; Falagan-Lotsch, P.; Lopes, T.S.; Granjeiro, J.M.; Amorim, L.M. Buccal cells DNA extraction to obtain high quality human genomic DNA suitable for polymorphism genotyping by PCR-RFLP and Real-Time PCR. J. Appl. Oral Sci. 2012, 20, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Luo, M.; Liang, G.; Pan, J.; Han, Y.; Zeng, L.; Yang, W.; Liu, J. What Factors Are Associated with Symptomatic Rotator Cuff Tears: A Meta-analysis. Clin. Orthop. Relat. Res. 2022, 480, 96–105. [Google Scholar] [CrossRef]
- Tooth, C.; Gofflot, A.; Schwartz, C.; Croisier, J.-L.; Beaudart, C.; Bruyère, O.; Forthomme, B. Risk Factors of Overuse Shoulder Injuries in Overhead Athletes: A Systematic Review. Sport. Health 2020, 12, 478–487. [Google Scholar] [CrossRef]
- Blonna, D.; Giani, A.; Bellato, E.; Mattei, L.; Caló, M.; Rossi, R.; Castoldi, F. Predominance of the critical shoulder angle in the pathogenesis of degenerative diseases of the shoulder. J. Shoulder Elb. Surg. 2016, 25, 1328–1336. [Google Scholar] [CrossRef]
- Gumina, S.; Arceri, V.; Carbone, S.; Albino, P.; Passaretti, D.; Campagna, V.; Fagnani, C.; Postacchini, F. The association between arterial hypertension and rotator cuff tear: The influence on rotator cuff tear sizes. J. Shoulder Elb. Surg. 2013, 22, 229–232. [Google Scholar] [CrossRef]
- Oliva, F.; Osti, L.; Padulo, J.; Maffulli, N. Epidemiology of the rotator cuff tears: A new incidence related to thyroid disease. Muscles Ligaments Tendons J. 2014, 4, 309–314. [Google Scholar] [CrossRef]
- Roy, J.S.; Braën, C.; Leblond, J.; Desmeules, F.; Dionne, C.E.; MacDermid, J.C.; Bureau, N.J.; Frémont, P. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterisation of rotator cuff disorders: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1316–1328. [Google Scholar] [CrossRef] [Green Version]
- Bishop, J.Y.; Santiago-Torres, J.E.; Rimmke, N.; Flanigan, D.C. Smoking predisposes to rotator cuff pathology and shoulder dysfunction: A systematic review. Arthroscopy 2015, 31, 1598–1605. [Google Scholar] [CrossRef]
- Bodin, J.; Ha, C.; Petit Le Manac’h, A.; Sérazin, C.; Descatha, A.; Leclerc, A.; Goldberg, M.; Roquelaure, Y. Risk factors for incidence of rotator cuff syndrome in a large working population. Scand. J. Work Environ. Health 2012, 38, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Plate, J.F.; Haubruck, P.; Walters, J.; Mannava, S.; Smith, B.P.; Smith, T.L.; Tuohy, C.J. Rotator cuff injuries in professional and recreational athletes. J. Surg. Orthop. Adv. 2013, 22, 134–142. [Google Scholar] [CrossRef]
- Cohen, C.; Figueiredo, E.A.; Belangero, P.S.; Andreoli, C.V.; Leal, M.F.; Ejnisman, B. Genetic aspects in shoulder disorders. Rev. Bras. Ortop. 2020, 55, 537–542. [Google Scholar] [CrossRef]
- Hoffecker, J.F.; Scott, A.E.; O’Rourke, D.H.; Scott, G.R.; Bigelow, N.H. Beringia and the global dispersal of modern humans. Evol. Anthropol. 2016, 25, 64–78. [Google Scholar] [CrossRef]
- Santos, N.P.C.; Ribeiro-Rodrigues, E.M.; Ribeiro-dos-Santos, A.K.C.; Pereira, R.; Gusmão, L.; Amorim, A.; Guerreiro, J.F.; Zago, M.A.; Matte, C.; Hutz, M.H.; et al. Assessing individual interethnic admixture and population substructure using a 48-insertion-deletion (INSEL) ancestry-informative marker (AIM) panel. Hum. Mutat. 2010, 31, 184–190. [Google Scholar] [CrossRef]
- Yuan, J.; Murrell, G.A.; Wei, A.Q.; Wang, M.X. Apoptosis in rotator cuff tendonopathy. J. Orthop. Res. 2002, 20, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Sejersen, M.H.; Frost, P.; Hansen, T.B.; Deutch, S.R.; Svendsen, S.W. Proteomics perspectives in rotator cuff research: A systematic review of gene expression and protein composition in human tendinopathy. PLoS ONE 2015, 10, e0119974. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Boosani, C.S.; Dilisio, M.F.; Gross, R.M.; Agrawal, D.K. Genes interconnecting AMPK and TREM-1 and associated microRNAs in rotator cuff tendon injury. Mol. Cell Biochem. 2019, 454, 97–109. [Google Scholar] [CrossRef] [PubMed]

| Variable | Case | Control | p Value 1 |
|---|---|---|---|
| Age | 0.0011 | ||
| >50 years | 3 (4.6%) | 21 (26.2%) | |
| <50 years | 62 (95.4%) | 59 (73.8%) | |
| Gender | 0.6283 | ||
| Female | 46 (57.5%) | 50 (62.5%) | |
| Male | 34 (42.5%) | 30 (37.5%) | |
| Self-reported ethnicity | 0.2639 | ||
| Yellow | 2 (2.5%) | 3 (3.9%) | |
| White | 29 (36.3%) | 27 (35.1%) | |
| Brown | 46 (57.5%) | 38 (49.3%) | |
| Black | 3 (3.7%) | 9 (11.7%) | |
| Overhead activities | 0.675 | ||
| No | 48 (64.8%) | 47 (60.3%) | |
| Yes | 26 (35.2%) | 31 (39.7%) | |
| Smoking | 0.532 | ||
| No | 76 (95%) | 73 (91.2%) | |
| Yes | 4 (5%) | 7 (8.8%) | |
| Alcoholism | 0.532 | ||
| No | 76 (95%) | 73 (91.2%) | |
| Yes | 4 (5%) | 7 (8.8%) | |
| Hypothyroidism | 1.000 2 | ||
| Absent | 74 (96.1%) | 76 (95%) | |
| Present | 3 (3.9%) | 4 (5%) | |
| Hypertension | 0.001 | ||
| Absent | 40 (50.6%) | 63 (79.7%) | |
| Present | 39 (49.4%) | 16 (20.3%) | |
| Tendinopathies | 0.002 | ||
| Absent | 59 (74.7%) | 73 (93.6%) | |
| Present | 20 (25.3%) | 5 (6.4%) |
| Genotype | Case N (%) | Control N (%) | OR | CI95% | p Value |
|---|---|---|---|---|---|
| GG 1 | 44 (73.3%) | 43 (55.1%) | 1.00 | 0.2821 | |
| AG | 12 (20.0%) | 19 (24.4%) | 0.64 | 0.26–1.61 | |
| AA | 4 (6.7%) | 16 (20.5%) | 0.40 | 0.11–1.44 | |
| GG 2 | 44 (73.3%) | 43 (55.1%) | 1.00 | 0.1464 | |
| AG + AA | 16 (26.7%) | 35 (44.9%) | 0.55 | 0.25–1.24 | |
| GG + AG 3 | 56 (93.3%) | 62 (79.5%) | 1.00 | ||
| AA | 4 (6.7%) | 16 (20.5%) | 0.46 | 0.13–1.59 | 0.2023 |
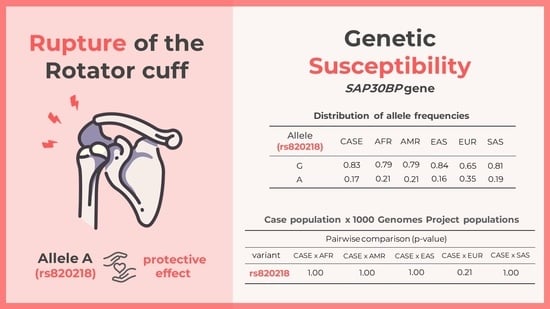
| Allele (rs820218) | CASE | AFR | AMR | EAS | EUR | SAS |
|---|---|---|---|---|---|---|
| G | 0.83 | 0.79 | 0.79 | 0.84 | 0.65 | 0.81 |
| A | 0.17 | 0.21 | 0.21 | 0.16 | 0.35 | 0.19 |
| Pairwise Comparison (p Value) | |||||
|---|---|---|---|---|---|
| Variant | CASE × AFR | CASE × AMR | CASE × EAS | CASE × EUR | CASE × SAS |
| rs820218 | 1.00 | 1.00 | 1.00 | 0.21 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, R.S.M.d.; Sant’ Anna, C.d.C.; Alcantara, D.D.F.Á.; Pantoja, K.B.C.C.; Fernandes, M.R.; Bentes, L.G.d.B.; Pimentel, A.L.J.C.; Lemos, R.S.; Almeida, N.R.C.d.; Fernandes, M.R.N.; et al. Association between the rs820218 Variant within the SAP30BP Gene and Rotator Cuff Rupture in an Amazonian Population. Genes 2023, 14, 367. https://doi.org/10.3390/genes14020367
Barros RSMd, Sant’ Anna CdC, Alcantara DDFÁ, Pantoja KBCC, Fernandes MR, Bentes LGdB, Pimentel ALJC, Lemos RS, Almeida NRCd, Fernandes MRN, et al. Association between the rs820218 Variant within the SAP30BP Gene and Rotator Cuff Rupture in an Amazonian Population. Genes. 2023; 14(2):367. https://doi.org/10.3390/genes14020367
Chicago/Turabian StyleBarros, Rui Sergio Monteiro de, Carla de Castro Sant’ Anna, Diego Di Felipe Ávila Alcantara, Karla Beatriz Cardias Cereja Pantoja, Marianne Rodrigues Fernandes, Lívia Guerreiro de Barros Bentes, Antônio Leonardo Jatahi Cavalcanti Pimentel, Rafael Silva Lemos, Nyara Rodrigues Conde de Almeida, Manuela Rodrigues Neiva Fernandes, and et al. 2023. "Association between the rs820218 Variant within the SAP30BP Gene and Rotator Cuff Rupture in an Amazonian Population" Genes 14, no. 2: 367. https://doi.org/10.3390/genes14020367