Genetic Improvement and Application Practices of Synthetic Hexaploid Wheat
Abstract
:1. Introduction
2. High Breeding Potential of Synthetic Hexaploid Wheat
3. Enhanced Genomic Variation and Recombination in Synthetic Hexaploid Wheat
4. A Case of Successful Direct Application of SHW: Chuanmai 42 from Southwestern China
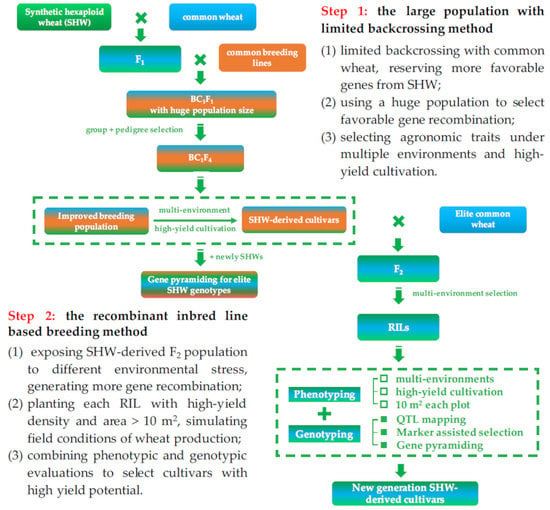
5. Further Application Using SHW-Derived Cultivars: Chuanmai 104
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Statistical Yearbook; FAO: Rome, Italy, 2017. [Google Scholar]
- Wang, J.; Luo, M.C.; Chen, Z.; You, F.M.; Wei, Y.; Zheng, Y.; Dvorak, J. Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol. 2013, 198, 925–937. [Google Scholar] [CrossRef]
- Ji, Y.; Chetelat, R.T. Homoeologous pairing and recombination in Solanum lycopersicoides monosomic addition and substitution lines of tomato. Theor. Appl. Genet. 2003, 106, 979–989. [Google Scholar] [CrossRef]
- Nagy, E.D.; Chu, Y.; Guo, Y.; Khanal, S.; Tang, S.; Li, Y.; Dong, W.B.; Timper, P.; Taylor, C.; Ozias-Akins, P.; et al. Recombination is suppressed in an alien introgression in peanut harboring Rma, a dominant root-knot nematode resistance gene. Mol. Breed. 2010, 26, 357–370. [Google Scholar] [CrossRef]
- Xie, W.; Ben-David, R.; Zeng, B.; Dinoor, A.; Xie, C.; Sun, Q.; Röder, M.S.; Fahoum, A.; Fahima, T. Suppressed recombination rate in 6VS/6AL translocation region carrying the Pm21 locus introgressed from Haynaldia villosa into hexaploid wheat. Mol. Breed. 2012, 29, 399–412. [Google Scholar] [CrossRef]
- Ren, T.; Li, Z.; Yan, B.; Tan, F.; Tang, Z.; Fu, S.; Yang, M.; Ren, Z. Targeted segment transfer from rye chromosome 2R to wheat chromosomes 2A, 2B, and 7B. Cytogenet. Genome Res. 2017, 151, 50–59. [Google Scholar] [CrossRef]
- Mujeeb-Kazi, A.; Rosas, V.; Roldan, S. Conservation of the genetic variation of Triticum tauschii (Coss.) Schmalh. (Aegilops squarrosa auct. non L.) in synthetic hexaploid wheats (T. turgidum L. s.lat. x T. tauschii; 2n = 6x = 42, AABBDD) and its potential utilization for wheat improvement. Genet. Resour. Crop Evol. 1996, 43, 129–134. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, G.; Yan, Z.; Chen, Q.; Yuan, Z.; Lan, X.; Zheng, Y.; Liu, D. Comparison of newly synthetic hexaploid wheat with its donors on SSR products. J. Genet. Genom. 2007, 34, 939–946. [Google Scholar] [CrossRef]
- Wan, H.; Li, J.; Ma, S.; Yang, F.; Chai, L.; Liu, Z.; Wang, Q.; Pu, Z.; Yang, W. Allopolyploidization increases genetic recombination in the ancestral diploid D genome during wheat evolution. Crop J. 2022, 10, 743–753. [Google Scholar] [CrossRef]
- Yang, F.; Wan, H.; Li, J.; Wang, Q.; Yang, N.; Zhu, X.; Liu, Z.; Yang, Y.; Ma, W.; Fan, X.; et al. Pentaploidization enriches the genetic diversity of wheat by enhancing the recombination of AB Genomes. Front. Plant Sci. 2022, 13, 883868. [Google Scholar] [CrossRef]
- Yang, W.; Liu, D.; Li, J.; Zhang, L.; Wei, H.; Hu, X.; Zheng, Y.; He, Z.; Zou, Y. Synthetic hexaploid wheat and its utilization for wheat genetic improvement in China. J. Genet. Genom. 2009, 36, 539–546. [Google Scholar] [CrossRef]
- Mujeeb-Kazi, A.; Kazi, A.G.; Dundas, I.; Rasheed, A.; Ogbonnaya, F.; Kishii, M.; Bonnett, D.; Wang, R.R.C.; Xu, S.; Chen, P.; et al. Genetic diversity for wheat improvement as a conduit to food security. Adv. Agron. 2013, 122, 179–257. [Google Scholar]
- Li, J.; Wan, H.S.; Yang, W.Y. Synthetic hexaploid wheat enhances variation and adaptive evolution of bread wheat in breeding processes. J. Syst. Evol. 2014, 52, 735–742. [Google Scholar] [CrossRef]
- Li, A.; Liu, D.; Yang, W.; Kishii, M.; Mao, L. Synthetic hexaploid wheat: Yesterday, today and tomorrow. Engineering 2018, 4, 552–558. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, L.; Zhao, L.; Dai, S.; Li, A.; Yang, W.; Xie, D.; Li, Q.; Ning, S.; Yan, Z.; et al. A breeding strategy targeting the secondary gene pool of bread wheat: Introgression from a synthetic hexaploid wheat. Theor. Appl. Genet. 2019, 132, 2285–2294. [Google Scholar] [CrossRef]
- Tang, Y.; Rosewarne, G.M.; Li, C.; Wu, X.; Yang, W.; Wu, C. Physiological factors underpinning grain yield improvements of synthetic-derived wheat in southwestern China. Crop Sci. 2015, 55, 98–112. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Li, C.; Yang, W.; Huang, M.; Ma, X.; Li, S. Yield, growth, canopy traits and photosynthesis in high-yielding, synthetic hexaploid-derived wheats cultivars compared with non-synthetic wheats. Crop Pasture Sci. 2017, 68, 115–125. [Google Scholar] [CrossRef]
- Liu, M.; Tong, H.; Liu, Y.; Li, C.; Wu, X.; Li, M.; Li, X.; Tang, Y. Genetic progress in grain yield and the associated physiological traits of popular wheat in southwestern China from 1969 to 2012. Crop Sci. 2021, 61, 1971–1986. [Google Scholar] [CrossRef]
- del Blanco, I.A.; Rajaram, S.; Kronstad, W.E.; Reynolds, M.P. Physiological performance of synthetic hexaploid wheat-derived populations. Crop Sci. 2000, 40, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, R.; Chen, Y.; Li, H.; Wei, T.; Xie, W.; Fan, G. Agronomic and physiological traits associated with genetic improvement of phosphorus use efficiency of wheat grown in a purple lithomorphic soil. Crop J. 2022, 10, 1151–1164. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Drought adaptive traits and wide adaptation in elite lines derived from resynthesized hexaploid wheat. Crop Sci. 2011, 51, 1617–1626. [Google Scholar] [CrossRef]
- Innes, R.L.; Kerber, E.R. Resistance to wheat leaf rust and stem rust in Triticum tauschii and inheritance in hexaploid wheat of resistance transferred from T. tauschii. Genome 1994, 37, 813–822. [Google Scholar] [CrossRef]
- Yang, W.Y.; Yu, Y.; Zhang, Y.; Hu, X.R.; Wang, Y.; Zhou, Y.C.; Lu, B.R. Inheritance and expression of stripe rust resistance in common wheat (Triticum aestivum) transferred from Aegilops tauschii and its utilization. Hereditas 2003, 139, 49–55. [Google Scholar] [CrossRef]
- Olson, E.L.; Rouse, M.N.; Pumphrey, M.O.; Bowden, R.L.; Gill, B.S.; Poland, J.A. Simultaneous transfer, introgression and genomic localization of genes for resistance to stem rust race TTKSK (Ug99) from Aegilops tauschii to wheat. Theor. Appl. Genet. 2013, 126, 1179–1188. [Google Scholar] [CrossRef]
- Miranda, L.M.; Murphy, J.P.; Marshall, D.; Leath, S. Pm34: A new powdery mildew resistance gene transferred from Aegilops tauschii Coss. to common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 1497–1504. [Google Scholar] [CrossRef]
- Wiersma, A.T.; Pulman, J.A.; Brown, L.K.; Cowger, C.; Olson, E.L. Identification of Pm58 from Aegilops tauschii. Theor. Appl. Genet. 2017, 130, 1123–1133. [Google Scholar] [CrossRef]
- Xue, S.; Hu, S.; Chen, X.; Ma, Y.; Lu, M.; Bai, S.; Wang, X.; Sun, T.; Wang, Y.; Wan, H.; et al. Fine mapping of Pm58 from Aegilops tauschii conferring powdery mildew resistance. Theor. Appl. Genet. 2022, 135, 1657–1669. [Google Scholar] [CrossRef]
- Cox, T.S.; Hatchett, J.H. Hessian fly-resistance gene H26 transferred from Triticum tauschii to common wheat. Crop Sci. 1994, 34, 958–960. [Google Scholar] [CrossRef]
- Zhu, L.; Smith, C.M.; Fritz, A.; Boyko, E.; Voothuluru, P.; Gill, B.S. Inheritance and molecular mapping of new greenbug resistance genes in wheat germplasms derived from Aegilops tauschii. Theor. Appl. Genet. 2005, 111, 831–837. [Google Scholar] [CrossRef]
- Ryan, P.R.; Raman, H.; Gupta, S.; Sasaki, T.; Yamamoto, Y.; Delhaize, E. The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J. 2010, 64, 446–455. [Google Scholar] [CrossRef]
- Sohail, Q.; Inoue, T.; Tanaka, H.; Eltayeb, A.E.; Matsuoka, Y.; Tsujimoto, H. Applicability of Aegilops tauschii drought tolerance traits to breeding of hexaploid wheat. Breed. Sci. 2011, 61, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Zhao, S.; Kong, X.; Li, Y.; Zhao, G.; He, W.; Appels, R.; Pfeifer, M.; Tao, Y.; Zhang, X.; et al. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 2013, 496, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, D.; Wu, J.; Zhao, X.; Hao, M.; Geng, S.; Yan, J.; Jiang, X.; Zhang, L.; Wu, J.; et al. mRNA and Small RNA transcriptomes reveal insights into dynamic homoeolog regulation of allopolyploid heterosis in nascent hexaploid wheat. Plant Cell 2014, 26, 1878–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.Q.; Kempf, H.; Ganal, M.W.; Röder, M.S. Advanced backcross QTL analysis in progenies derived from a cross between a German elite winter wheat variety and a synthetic wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kulwal, P.L.; Balyan, H.S.; Gupta, P.K. QTL mapping for yield and yield contributing traits in two mapping populations of bread wheat. Mol. Breed. 2007, 19, 163–177. [Google Scholar] [CrossRef]
- Yu, M.; Chen, G.; Zhang, L.; Liu, Y.; Liu, D.; Wang, J.; Pu, Z.; Zhang, L.; Lan, X.; Wei, Y.; et al. QTL mapping for important agronomic traits in synthetic hexaploid wheat derived from Aegiliops tauschii ssp. tauschii. J. Integr. Agric. 2014, 13, 1835–1844. [Google Scholar] [CrossRef]
- Wan, H.; Yang, Y.; Li, J.; Zhang, Z.; Yang, W. Mapping a major QTL for hairy leaf sheath introgressed from Aegilops tauschii and its association with enhanced grain yield in bread wheat. Euphytica 2015, 205, 275–285. [Google Scholar] [CrossRef]
- Dvorak, J.; Luo, M.C.; Yang, Z.L.; Zhang, H.B. The structure of the Aegilops tauschii genepool and the evolution of hexaploid wheat. Theor. Appl. Genet. 1998, 97, 657–670. [Google Scholar] [CrossRef]
- Dvorak, J.; Deal, K.R.; Luo, M.C.; You, F.M.; von Borstel, K.; Dehghani, H. The origin of spelt and free-threshing hexaploid wheat. J. Hered. 2012, 103, 426–441. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhao, X.; Li, Y.; Xu, J.; Bi, A.; Kang, L.; Xu, D.; Chen, H.; Wang, Y.; Wang, Y.G.; et al. Triticum population sequencing provides insights into wheat adaptation. Nat. Genet. 2020, 52, 1412–1422. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploidy. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Hao, M.; Luo, J.; Zeng, D.; Zhang, L.; Ning, S.; Yuan, Z.; Yan, Z.; Zhang, H.; Zheng, Y.; Feuillet, C.; et al. QTug.sau-3B is a major quantitative trait locus for wheat hexaploidization. Genes Genomes Genet. 2014, 4, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Fedak, G.; Guo, W.; Liu, B. Rapid and repeatable elimination of a parental genome-specific DNA repeat (pGc1R-1a) in newly synthesized wheat allopolyploids. Genetics 2005, 170, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.A.; Guan, L.L.; Chen, G.Y.; Pu, Z.E.; Hou, D.B. Allopolyploidy-induced rapid genomic changes in newly generated synthetic hexaploid wheat. Biotechnol. Biotechnol. Equip. 2017, 31, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhao, L.; Zheng, J.; Li, Y.; Zhang, L.; Liu, D.; Pu, Z.; Hao, M. Karyotype mosaicism in early generation synthetic hexaploid wheats. Genome 2020, 63, 329–336. [Google Scholar] [CrossRef] [PubMed]
- The Wheat Yield per mu in Jiangyou Reach a New Record in Sichuan Province (In Chinese). Available online: http://www.moa.gov.cn/ztzl/qnszcgdfs/201006/t20100602_1497968.htm (accessed on 2 June 2010).
- Representative Achievements: Exploration of Superior Gene from Synthetic Hexaploid wheat and Breeding and Popularization of Chuanmai 42-Series Cultivars (In Chinese). Available online: http://www.chinawestagr.com/zwyjs/showcontent.asp?id=49833 (accessed on 1 September 2022).
- Liao, J.; Wei, H.T.; Li, J.; Yang, Y.M.; Zeng, Y.C.; Peng, Z.S.; Yang, W.Y. Detection of the introgression loci of synthetic hexaploid wheat in wheat variety Chuanmai 42 by SSR markers. Acta Agron. Sin. 2007, 33, 703–707, (In Chinese with English Abstract). [Google Scholar]
- Li, J.; Wei, H.T.; Hu, X.R.; Li, C.S.; Tang, Y.L.; Liu, D.C.; Yang, W.Y. Identification of a high-yield introgression locus in Chuanmai 42 inherited from synthetic hexaploid wheat. Acta Agron. Sin. 2011, 37, 255–262. [Google Scholar] [CrossRef]
- Li, G.Q.; Li, Z.F.; Yang, W.Y.; Zhang, Y.; He, Z.H.; Xu, S.C.; Singh, R.P.; Qu, Y.Y.; Xia, X.C. Molecular mapping of stripe rust resistance gene YrCH42 in Chinese wheat cultivar Chuanmai42 and its allelism with Yr24 and Yr26. Theor. Appl. Genet. 2006, 112, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, H.S.; Yang, W.Y.; Wang, Q.; Zhu, X.G.; Hu, X.R.; Wei, H.T.; Tang, Y.L.; Li, C.S.; Peng, Z.S.; et al. Dissection of genetic components in the new high-yielding wheat cultivar Chuanmai 104. Sci. Agric. Sin. 2014, 47, 2281–2291, (In Chinese with English abstract). [Google Scholar]
- Chuanmai 104, Bred by Sichuan Academy of Agricultural Sciences, Reached the Grain Yield of 729.8 kg/mu, Breaking the Record of Wheat Yield per mu in Southwestern China (In Chinese). Available online: http://www.chinawestagr.com/homepage/showcontent.asp?id=40323 (accessed on 19 May 2020).
- Decoding Science & Technology of Sichuan II: What Is the New Cultivar Chuanmai 104 with the Maximum Grain Yield 729.8 kg/mu? Let’s Find Out (in Chinese). Available online: https://cbgc.scol.com.cn/news/1005312 (accessed on 17 March 2021).
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef]
- Phillips, D.; Jenkins, G.; Macaulay, M.; Nibau, C.; Wnetrzak, J.; Fallding, D.; Colas, I.; Oakey, H.; Waugh, R.; Ramsay, L. The effect of temperature on the male and female recombination landscape of barley. New Phytol. 2015, 208, 421–429. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J.; Huang, J.; Cui, K.; Wang, F.; Douthe, C.; Lin, M. Why high yield QTLs failed in preventing yield stagnation in rice? Crop Environ. 2022, 1, 103–107. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.H.; Wan, H.S.; Wei, H.T.; Long, H.; Li, T.; Deng, G.B.; Li, J.; Yang, W.Y. Identification and pyramiding of QTLs for traits associated with pre-harvest sprouting resistance in two wheat cultivars Chuanmai 42 and Chuannong 16. Sci. Agric. Sin. 2020, 53, 3421–3431, (In Chinese with English abstract). [Google Scholar]
- Yang, M.; Li, G.; Wan, H.; Li, L.; Li, J.; Yang, W.; Pu, Z.; Yang, Z.; Yang, E. Identification of QTLs for stripe rust resistance in a recombinant inbred line population. Int. J. Mol. Sci. 2019, 20, 3410. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, Q.; Wan, H.; Yang, F.; Wei, H.; Xu, Z.; Ji, H.; Xia, X.; Li, J.; Yang, W. QTL mapping for adult-plant resistance to powdery mildew in Chinese elite common wheat Chuanmai 104. Cereal Res. Commun. 2021, 49, 99–108. [Google Scholar] [CrossRef]
- Tang, Y.L.; Li, J.; Wu, Y.Q.; Wei, H.T.; Li, C.S.; Yang, W.Y.; Chen, F. Identification of QTLs for yield-related traits in the recombinant inbred line population derived from the cross between a synthetic hexaploid wheat derived variety Chuanmai 42 and a Chinese elite variety Chuannong 16. Agric. Sci. Chi. 2011, 10, 1665–1680. [Google Scholar] [CrossRef]
- Lu, B.; Deng, G.; Zhang, H.; Li, J.; Wan, H.; Pan, Z.; Yang, W.; Yu, M.; Long, H. QTL mapping of yield-related traits in the high-yield wheat variety Chuanmai42. Chin. J. Appl. Environ. Biol. 2017, 23, 0183–0192, (In Chinese with English abstract). [Google Scholar]
- Zhu, X.; Wan, H.; Li, J.; Zheng, J.; Tang, Z.; Yang, W. Mixed major-genes plus polygenes inheritance analysis for breeding superiority in synthetic hexaploid wheat. J. Nanjing Agric. Univ. 2018, 41, 625–632, (In Chinese with English abstract). [Google Scholar]
- Yang, Y.; Wan, H.; Yang, F.; Xiao, C.; Li, J.; Ye, M.; Chen, C.; Deng, G.; Wang, Q.; Li, A.; et al. Mapping QTLs for enhancing early biomass derived from Aegilops tauschii in synthetic hexaploid wheat. PloS ONE 2020, 15, e0234882. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, N.; Majidi, M.M.; Mirlohi, A. Potentials of synthetic hexaploid wheats to improve drought tolerance. Sci. Rep. 2022, 12, 20482. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.M.I.; Morgounov, A.; Baenziger, P.S.; Esmail, S.M. Genetic variation in common bunt resistance in synthetic hexaploid wheat. Plants 2023, 12, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Jia, X.; Tian, Z.; Wang, Y.; Wang, C.; Zhang, H.; Liu, X.; Zhao, J.; Deng, P.; et al. Chromosome karyotype and stability of new synthetic hexaploid wheat. Mol. Breeding 2021, 41, 60. [Google Scholar] [CrossRef]
- Gorafi, Y.S.A.; Kim, J.S.; Elbashir, A.A.E.; Tsujimoto, H. A population of wheat multiple synthetic derivatives: An effective platform to explore, harness and utilize genetic diversity of Aegilops tauschii for wheat improvement. Theor. Appl. Genet. 2018, 131, 1615–1626. [Google Scholar] [CrossRef] [PubMed]







| SHWs | Sub-Genome | Genomic Variation | S0 | S6 |
|---|---|---|---|---|
| Langdon/SQ783 | AB genome | SNP | 242,260 | 10,609,935 |
| Deletion | 25,376 | 354,762 | ||
| Insertion | 8017 | 346,855 | ||
| Total | 275,653 | 11,311,552 | ||
| D genome | SNP | 40,365 | 5,610,199 | |
| Deletion | 8121 | 263,781 | ||
| Insertion | 8021 | 294,170 | ||
| Total | 56,507 | 6,168,150 | ||
| Langdon/SQ665 | AB genome | SNP | 39,185 | 235,260 |
| Deletion | 6697 | 25,156 | ||
| Insertion | 6674 | 7784 | ||
| Total | 52,556 | 268,200 | ||
| D genome | SNP | 27,691 | 3,906,319 | |
| Deletion | 4763 | 212,619 | ||
| Insertion | 4764 | 186,499 | ||
| Total | 37,218 | 4,305,437 |
| Year | Trial Grade | Average Yield (Kg/mu) a | Check Cultivar | Increasing Rate (%) |
|---|---|---|---|---|
| 2002 | Sichuan regional cultivar trials | 414.6 | Chuanmai 28 | 70.2 b |
| 2003 | Sichuan regional cultivar trials | 403.2 | Chuanmai 107 | 28.3 |
| 2003 | National regional cultivar trials | 354.7 | Chuanmai 107 | 16.3 |
| 2004 | National regional cultivar trials | 406.3 | Chuanmai 107 | 16.5 |
| Cultivar | Pedigree a | Generation | Regional Trial Yield (Kg/mu) | Check Cultivar | Increasing Rate (%) | Released Time |
|---|---|---|---|---|---|---|
| Chuanmai 51 | 174/183//99-1572 | 1st | 373.9 | Chuanmai 107 | 13.4 | 2008 |
| Chuanmai 56 | Chuanmai 30/Chuanmai 42 | 1st | 362.7 | Chuanmai 107 | 13.5 | 2009 |
| Chuanmai 58 | Chuanmai 42/03Jian3//Chuanmai 42 | 1st | 381.9 | Mianmai 37 | 5.0 | 2010 |
| Chuanmai 104 | Chuanmai 42/Chuannong 16 | 1st | 408.7 | Chuanmai 42 | 8.5 | 2012 |
| Shumai 969 | SHW-L1/SW8188//Chuanyu 18/3/Chuanmai 42 | 1st | 384.0 | Mianmai 37 | 8.1 | 2013 |
| Chuanmai 64 | Chuanmai 42/Chuannong 16 | 1st | 400.3 | Mianmai 37 | 12.0 | 2013 |
| Chuanmai 90 | Jian38/99116//Chuanmai 42 | 1st | 371.1 | Mianmai 37 | 6.9 | 2014 |
| Chuanmai 91 | Neimai 8/Zhengmai 9023//00062/3/Chuanmai 42 | 1st | 375.4 | Mianmai 37 | 5.4 | 2014 |
| Zhongkemai 138 | Chuanmai 42/Chuanyu 16 | 1st | 389.0 | Mianmai 37 | 12.0 | 2014 |
| Chuanmai 92 | Neimai 8/Jian3//Chuanmai 42 | 1st | 353.0 | Mianmai 37 | 11.7 | 2015 |
| Chuanmai 81 | SW8019/99-1572//99-1572 | 1st | 350.4 | Mianmai 37 | 8.1 | 2015 |
| Guohaomai 3 | 1227-185/99-1522//99-1572 | 1st | 384.0 | Chuanmai 42 | −-3.5 | 2016 |
| Chuanmai 602 | Guinong 21/SW324/Chuanmai 42 | 1st | 403.5 | Mianmai 367 | 13.0 | 2017 |
| Chuanmai 603 | Aibaiguinong 21/Mianyang 26//Chuanmai 42 | 1st | 355.1 | Mianmai 367 | 6.3 | 2018 |
| Chuanmai 604 | Guinong 21/SW3243//Chuanmai 42 | 1st | 388.2 | Mianmai 367 | 13.0 | 2018 |
| Chuanmai 86 | R4117/1572 | 1st | 382.8 | Mianmai 367 | 12.5 | 2018 |
| Neimai 101 | 99-1572/M0501 | 1st | 394.8 | Mianmai 367 | 10.5 | 2020 |
| Chuanmai 53 | 477(99-1572)/Miannong 4//Y314 | 2nd | 351.1 | Chuanmai 107 | 20.2 | 2009 |
| Chuanmai 66 | 99-1572/98-266//01-3570 | 2nd | 377.3 | Mianmai 37 | 5.6 | 2014 |
| Chuanmai 67 | 99-1572/SW8688//01-3570 | 2nd | 391.6 | Mianmai 37 | 9.9 | 2014 |
| Zhongkemai 169 | Zhongkemai 138/Chuannong 27 | 2nd | 380.7 | Mianmai 367 | 7.8 | 2019 |
| Zhongkemai 13 | R64002/Zhongkemai 138 | 2nd | 389.0 | Mianmai 367 | 7.0 | 2020 |
| Zhongke-NM 168 | Zhongkemai 138/PW18 | 2nd | 221.1 | Yumai 13 | 2.3 | 2020 |
| Chuanmai 68 | 99-1572/98-266//01-3570 | 2nd | 379.6 | Mianmai 37 | 16.5 | 2015 |
| Chuanmai 601 | Guinong 21/SW3243//Chuanmai 42/ Chuanmai 44 | 2nd | 394.3 | Chuanmai 42 | 5.8 | 2018 |
| Xikemai 546 | 07Jian3401-05/Yumai 1 | 2nd | 381.4 | Mianmai 367 | 5.8 | 2021 |
| Cultivar | Pedigree a | Generation | Regional Trial Yield (Kg/mu) | Check Cultivar | Increasing Rate (%) | Released Time |
|---|---|---|---|---|---|---|
| Chuanmai 69 | Chuanmai 104/B2183 | 1st | 361.6 | Mianmai 37 | 13.2 | 2015 |
| Chuanmai 1546 | Chuanchongzu 104/Chuan 07005 | 1st | 374.8 | Mianmai 367 | 9.1 | 2018 |
| Chuanmai 93 | Pubing 3504/Chuanyu 20//Chuanmai 104 | 1st | 385.7 | Mianmai 367 | 16.9 | 2018 |
| Chuanmai 96 | Jian3/Chuannong 19//Chuanmai 104 | 1st | 380.9 | Mianmai 367 | 10.1 | 2018 |
| Chumai 16 | Neimai 8/Jian3//Chuanchongzu 104 | 1st | 437.2 | Yunmai 56 | 9.3 | 2018 |
| Chuanmai 1580 | Chuanchongzu 104/Chuan 07005 | 1st | 394.3 | Mianmai 367 | 10.4 | 2019 |
| Chuanmai 98 | Jian 3/Chuannong 19//Chuanmai 104 | 1st | 405.9 | Mianmai 367 | 13.0 | 2019 |
| Chuanmai 1648 | Chuanchongzu 104/CN16Xuan-1 | 1st | 420.0 | Mianmai 367 | 13.8 | 2020 |
| Chuanmai 1603 | Chuanchongzu 104/CN16Xuan-1 | 1st | 417.6 | Mianmai 367 | 15.9 | 2020 |
| Chuanmai 1694 | Chuanchongzu 104/Chuan 07005 | 1st | 399.7 | Mianmai 367 | 8.8 | 2020 |
| Neimai 866 | Chuanchongzu 104/Chuan 08Ping32 | 1st | 390.3 | Mianmai 367 | 7.5 | 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Yang, F.; Li, J.; Wang, Q.; Liu, Z.; Tang, Y.; Yang, W. Genetic Improvement and Application Practices of Synthetic Hexaploid Wheat. Genes 2023, 14, 283. https://doi.org/10.3390/genes14020283
Wan H, Yang F, Li J, Wang Q, Liu Z, Tang Y, Yang W. Genetic Improvement and Application Practices of Synthetic Hexaploid Wheat. Genes. 2023; 14(2):283. https://doi.org/10.3390/genes14020283
Chicago/Turabian StyleWan, Hongshen, Fan Yang, Jun Li, Qin Wang, Zehou Liu, Yonglu Tang, and Wuyun Yang. 2023. "Genetic Improvement and Application Practices of Synthetic Hexaploid Wheat" Genes 14, no. 2: 283. https://doi.org/10.3390/genes14020283