Karyotypes of Manatees: New Insights into Hybrid Formation (Trichechus inunguis × Trichechus m. manatus) in the Amazon Estuary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chromosomal Preparations and Karyotype Analysis
2.3. DNA Extraction and Isolation of Repetitive Sequences
2.4. Sequence Analysis
2.5. Fluorescent In Situ Hybridization (FISH)
3. Results
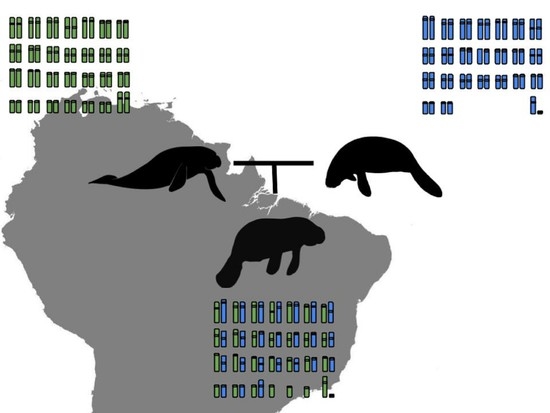
3.1. Karyotype Description and Comparisons
3.2. Mapping of Repetitive DNA Sequences
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonvicino, C.; Viana, M.C.; Oliveira, E.; Emin, R.; Silva Junior, J.; Sousa, M.; Siciliano, S. Distribution of South American manatees, Trichechus manatus Linnaeus, 1758 and T. inunguis (Natterer, 1883) (Sirenia: Trichechidae). Bol. Mus. Para. Emílio Goeldi. Sér. Ciênc. Nat. 2020, 15, 573–599. [Google Scholar] [CrossRef]
- Castelblanco-Martínez, D.N.; Morales-Vela, B.; Padilla-Saldívar, J.A. Using craniometrical predictors to infer body size of Antillean manatees. Mammalia 2014, 78, 109–115. [Google Scholar] [CrossRef]
- Assis, M.F.L.; Best, R.C.; Barros, R.M.S.; Yonenaga-Yassuda, Y. Cytogenetic study of Trichechus inunguis (Amazonian manatee). Braz. J. Genet. 1988, 11, 41–50. [Google Scholar]
- Gray, B.A.; Zori, R.T.; Mcguire, P.M.; Bonde, R.K. A first generation cytogenetic ideogram for the Florida manatee (Trichechus manatus latirostris) based on multiple chromosome banding techniques. Hereditas 2002, 137, 215–223. [Google Scholar] [CrossRef]
- Hunter, M.E.; Mignucci-Giannoni, A.A.; Tucker, K.P.; King, T.L.; Bonde, R.K.; Gray, B.A.; Mcguire, P.M. Puerto Rico and Florida manatees represent genetically distinct group. Conserv. Genet. 2012, 13, 1623–1635. [Google Scholar] [CrossRef]
- Kellogg, M.E.; Burkett, S.; Dennis, T.R.; Stone, G.; Gray, B.A.; Mcguire, P.M.; Zori, R.T.; Stanyon, R. Chromosome painting in the manatee supports Afrotheria and Paenungulata. BMC Evol. Biol. 2007, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.S.; Magalhães, R.F.; Marmontel, M.; Meirelles, A.C.; Carvalho, V.L.; Lavergne, A.; Thoisy, B.; Santos, F.R. A hybrid swarm of manatees along the Guianas coastline, a peculiar environment under the influence of the Amazon River plume. An. Acad. Bras. Cienc. 2019, 91, e20190325. [Google Scholar] [CrossRef]
- Vianna, J.A.; Bonde, R.K.; Caballero, S.; Giraldo, J.P.; Lima, R.P.; Clarl, A.; Marmontel, M.; Morales-Vela, B.; Souza, M.J.; Parr, L.; et al. Phylogeography, phylogeny and hybridization in trichechid sirenians: Implications for manatee conservation. Mol. Ecol. 2006, 15, 433–447. [Google Scholar] [CrossRef]
- De Oliveira, E.H.C.; Gomes, A.J.B.; Costa, A.F.; Emin-Lima, R.; Bonvicino, C.R.; Viana, M.C.; Reis, L.M.A.; Vidal, M.D.; Cavalcanti, M.V.G.; Attademo, F.L.N.; et al. Karyotypical confirmation of natural hybridization between two manatee species, Trichechus manatus and Trichechus inunguis. Life 2022, 12, 616. [Google Scholar] [CrossRef]
- Luna, F.O.; Beaver, C.E.; Nourisson, C.; Bonde, R.K.; Attademo, F.L.N.; Miranda, A.V.; Torres-Florez, J.P.; de Sousa, G.P.; Passavante, J.Z.; Hunter, M.E. Genetic connectivity of the west indian manatee in the southern range and limited evidence of hybridization with Amazonian manatees. Front. Mar. Sci. 2021, 7, 574455. [Google Scholar] [CrossRef]
- Heyduk, K.; McAssey, E.V.; Grimwood, J.; Shu, S.; Schmutz, J.; McKain, M.R.; Leebens-Mack, J. Hybridization history and repetitive element content in the genome of a homoploid hybrid, Yucca gloriosa (Asparagaceae). Front. Plant Sci. 2021, 11, 573767. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, B.; Snlegowshi, P.; Stephan, W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 1994, 371, 215–220. [Google Scholar] [CrossRef]
- Valeri, M.P.; Dias, G.B.; Espírito Santo, A.A.; Moreira, C.N.; Yonenaga-Yassuda, Y.; Sommer, I.B.; Kuhn, G.C.S.; Svartman, M. First description of a satellite DNA in manatees’ centromeric regions. Front. Genet. 2021, 12, 694866. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.D.; Dobigny, G.; Pardini, A.T.; Robinson, T.J. LINE1 distribution in Afrotheria and Xenarthra: Implications for understanding the evolution of LINE-1 in eutherian genomes. Chromosoma 2004, 113, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Paun, O.; Fay, M.F.; Soltis, D.E.; Chase, M.W. Genetic and epigenetic alterations after hybridization and genome doubling. Taxon 2007, 56, 649–656. [Google Scholar] [CrossRef]
- Nei, M.; Rooney, A.P. Concerted and Birth-and-Death Evolution of Multigene Families. Ann. Rev. Gen. 2005, 39, 125–152. [Google Scholar] [CrossRef] [Green Version]
- Ubeda-Manzanaro, M.; Merlo, M.A.; Palazon, J.L.; Cross, I.; Sarasquete, C.; Rebordinos, L. Chromosomal mapping of the major and minor ribosomal genes, (GATA)n and U2 snRNA gene by double-colour FISH in species of the Batrachoididae family. Genetica 2010, 138, 787–794. [Google Scholar] [CrossRef]
- Moorhead, P.S.; Nowell, P.C.; Mellmam, W.J.; Battips, D.M.; Hungerford, D.A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp. Cell Res. 1960, 20, 613–616. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Seabright, M. A rapid banding technique for human chromosomes. Lancet 1971, 2, 971–972. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandrerg, A.A. Nomeclature for centromereric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Ijdo, J.W.; Wells, R.A.; Baldini, A.; Reeders, S.T. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleics Acids Res. 1991, 19, 4780. [Google Scholar] [CrossRef] [Green Version]
- Cabral-De-Melo, D.C.; Moura, R.C.; Martins, C. Chromosomal mapping of repetitive DNAs in the beetle Dichotomius geminates provide the first evidence for an association of 5S rRNA and histone H3 genes in insects, and repetitive DNA similarity between the B chromosome and A complement. Heredity 2010, 104, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Colgan, D.J.; Mclauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Austral. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Gerlach, W.L.; Bedbrook, J.R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979, 7, 1869–1885. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Kohany, O.; Gentles, A.J.; Hankus, L.; Jurka, J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinform. 2006, 7, 474. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Alkalaeva, E.Z.; Perelman, P.L.; Pardini, A.T.; Harrison, W.R.; O’Brien, P.C.M.; Fu, B.; Graphodatsky, A.S.; Ferguson-Smith, M.A.; Robinson, T.J. Reciprocal chromosome painting among human, aardvark, and elephant (superorder Afrotheria) reveals the likely eutherian ancestral karyotype. Proc. Natl. Acad. Sci. USA 2003, 4, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Barros, H.; Meirelles, A.; Luna, F.; Marmontel, M.; Cordeiro-Estrela, P.; Santos, N.; Astúa, D. Cranial and chromosomal geographic variation in manatees (Mammalia: Sirenia: Trichechidae) with the description of the Antillean manatee karyotype in Brazil. J. Zool. Syst. Evol. Res. 2017, 55, 73–87. [Google Scholar] [CrossRef]
- Loughman, W.; Frye, F.; Herald, E. The chromosomes of a male manatee. Int. Zoo 1970, 10, 151–152. [Google Scholar] [CrossRef]
- Domning, D.P. A phylogenetic analysis of the Sirenia. In Contributions in Marine Mammal Paleontology Honoring, Proceedings of the San Diego Society of Natural History; Frank, C.W., Jr., Berta, A., Deméré, T.A., Eds.; Taylor & Francis, Ltd.: Abingdon, CA, USA, 1994; Volume 29, pp. 177–189. [Google Scholar]
- Meyne, J.; Baker, R.J.; Hobart, H.H.; Hsu, T.C.; Ryder, O.A.; Ward, O.G.; Wiley, J.E.; Wurster-Hill, D.H.; Yates, T.L.; Moyzis, R.K. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 1990, 99, 3–10. [Google Scholar] [CrossRef]
- Nagamachi, C.Y.; Pieczarka, J.C.; O’Brien, P.C.M.; Pinto, J.A.; Malcher, S.M.; Pereira, A.L.; Rissino, J.D.; Mendes-Oliveira, A.C.; Rossi, R.V.; Ferguson-Smith, M.A. FISH with whole chromosome and telomeric probes demonstrates huge karyotypic reorganization with ITS between two species of Oryzomyini (Sigmodontinae, Rodentia): Hylaeamys megacephalus probes on Cerradomys langguthi karyotype. Chromosome Res. 2013, 21, 107–111. [Google Scholar] [CrossRef]
- Grize, S.A.; Wilwert, E.; Searle, J.B.; Lindholm, A.K. Measurements of hybrid fertility and a test of mate preference for two house mouse races with massive chromosomal divergence. BMC Evol. Biol. 2019, 19, 25. [Google Scholar] [CrossRef]
- Belonogova, N.M.; Polyakov, A.V.; Karamysheva, T.V.; Torgasheva, A.A.; Searle, J.B.; Borodin, P.M. Chromosome synapsis and recombination in male hybrids between two chromosome races of the common shrew (Sorex araneus L., Soricidae, Eulipotyphla). Genes 2017, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Kang, H.; Sparkes, D.L.; Tao, S.; Fan, X.M.; Xu, L.; Fan, X.; Sha, L.; Zhang, H.; Wang, Y.; et al. Mitotic and meiotic behavior of rye chromosomes in wheat-Psathyrostachys huashanica amphiploid x triticale progeny. Genet. Mol. Res. 2013, 12, 2537–2548. [Google Scholar] [CrossRef]
- Poignet, M.; Johnson Pokorná, M.; Altmanová, M.; Majtánová, Z.; Dedukh, D.; Albrecht, T.; Reif, J.; Osiejuk, T.S.; Reifová, R. Comparison of Karyotypes in Two Hybridizing Passerine Species: Conserved Chromosomal Structure but Divergence in Centromeric Repeats. Front. Genet. 2021, 12, 768987. [Google Scholar] [CrossRef]
- Dedukh, D.; Krasikova, A. Delete and survive: Strategies of programmed genetic material elimination in eukaryotes. Biol. Rev. 2022, 97, 195–216. [Google Scholar] [CrossRef]
- Arkhipova, I.R.; Rodriguez, F. Genetic and epigenetic changes involving (retro)transposons in animal hybrids and polyploids. Cytogenet. Genome Res. 2013, 140, 295–311. [Google Scholar] [CrossRef]
- Ribeiro, L.B.; Moraes-Neto, A.; Artoni, R.F.; Matoso, D.A.; Feldberg, E. Chromosomal Mapping of Repetitive Sequences (Rex3, Rex6, and rDNA Genes) in Hybrids Between Colossoma macropomum (Cuvier, 1818) and Piaractus mesopotamicus (Holmberg, 1887). Zebrafish 2017, 14, 155–160. [Google Scholar] [CrossRef]
- O’Neill, R.J.W.; O’Neill, M.J.; Graves, J.A.M. Undermethylation associated with retroelement activation and chromosome remodelling in aninterspecific mammalian hybrid. Nature 1998, 393, 68–72. [Google Scholar] [CrossRef]
- Fontdevila, A. hybrid genome evolution by transposition: An update. J. Hered. 2019, 110, 124–136. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.J.; Eldridge, M.D.; Graves, J.A. Chromosome heterozygosity and de novo chromosome rearrangements in mammalian interspecies hybrids. Mamm. Genome 2001, 12, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.C.; Eler, E.S.E.; Silva, C.E.F.; da Silva, M.N.F.; Araújo, N.P.; Svartman, M.; Feldberg, E. LINE-1 and SINE-B1 mapping and genome diversification in Proechimys species (Rodentia: Echimyidae). Life Sci. Alliance 2022, 5, e202101104. [Google Scholar] [CrossRef]
- De Sotero-Caio, C.G.; Cabral-de-Mello, D.C.; Calixto, M.S.; Valente, G.T.; Martins, C.; Loreto, V.; Souza, M.J.; Santos, N. Centromeric enrichment of LINE-1 retrotransposons and its significance for the chromosome evolution of Phyllostomid bats. Chromosome Res. 2017, 25, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Molina, W.F.; Yano, C.F.; Zhang, Y.; de Oliveira, E.A.; Lou, B.; de Bello Cioffi, M. Comparative cytogenetics in three Sciaenid species (Teleostei, Perciformes): Evidence of interspecific chromosomal diversification. Mol. Cytogenet. 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.K.; Fujiwara, H. Cross-genome screening of novel sequence-specific non-LTR retrotransposons: Various multicopy RNA genes and microsatellites are selected as targets. Mol. Biol. Evol. 2004, 21, 207–217. [Google Scholar] [CrossRef]
- Pucci, M.B.; Nogaroto, V.; Moreira-Filho, O.; Vicari, M.R. Dispersion of transposable elements and multigene families: Microstructural variation in Characidium (Characiformes: Crenuchidae) genomes. Genet. Mol. Biol. 2018, 41, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Kojima, K.K.; Jurka, J. Ancient origin of the U2 small nuclear RNA gene- targeting non-LTR retrotransposons Utopia. PLoS ONE 2015, 10, e0140084. [Google Scholar] [CrossRef] [Green Version]
- Anjos, A.; Ruiz-Ruano, F.J.; Camacho, J.P.; Loreto, V.; Cabrero, J.; de Souza, M.J.; Cabral-de-Mello, D.C. U1 snDNA clusters in grasshoppers: Chromosomal dynamics and genomic organization. Heredity 2015, 114, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, N.L.; Cabral-de-Mello, D.C.; Rocha, M.F.; Loreto, V.; Martins, C.; Moura, R.C. Chromosomal mapping of rDNAs and H3 histone sequences in the grasshopper rhammatocerus brasiliensis (acrididae, gomphocerinae): Extensive chromosomal dispersion and co-localization of 5S rDNA/H3 histone clusters in the A complement and B chromosome. Mol. Cytogenet. 2011, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, D.T.; Ferguson-Smith, M.A.; Rens, W.; Foresti, F.; Porto-Foresti, F. Chromosome mapping of H1 histone and 5S rRNA gene clusters in three species of Astyanax (Teleostei, Characiformes). Cytogenet. Genome Res. 2011, 134, 64–71. [Google Scholar] [CrossRef]
- Pritham, E.J.; Feschotte, C. Massive amplification of rolling-circle transposons in the lineage of the bat Myotis lucifugus. Proc. Natl Acad. Sci. USA 2007, 104, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Phillips, C.D.; Baker, R.J.; Pritham, E.J. Rolling-circle transposons catalyze genomic innovation in a Mammalian lineage. Genome Biol. Evol. 2014, 6, 2595–2610. [Google Scholar] [CrossRef] [Green Version]
- Garrigues, J.M.; Tsu, B.V.; Daugherty, M.D.; Pasquinelli, A.E. Diversification of the Caenorhabditis heat shock response by Helitron transposable elements. Elife 2019, 8, e51139. [Google Scholar] [CrossRef]
- Lai, J.; Li, Y.; Messing, J.; Dooner, H.K. Gene movement by Helitron transposons contributes to the haplotype variability of maize. Proc. Natl. Acad. Sci. USA 2005, 102, 9068–9073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabundzija, I.; Messing, S.A.; Thomas, J.; Cosby, R.L.; Bilic, I.; Miskey, C.; Gogol-Döring, A.; Kapitonov, V.; Diem, T.; Dalda, A.; et al. A Helitron transposon reconstructed from bats reveals a novel mechanism of genome shuffling in eukaryotes. Nat. Commun. 2016, 7, 10716. [Google Scholar] [CrossRef] [Green Version]
- Almeida, B.R.R.; Milhomem-Paixão, S.S.R.; Noronha, R.C.R.; Nagamachi, C.Y.; Costa, M.J.R.; Pardal, P.P.O.; Coelho, J.S.; Pieczarka, J.C. Karyotype diversity and chromosomal organization of repetitive DNA in Tityus obscurus (Scorpiones, Buhidae). BMC Genet. 2017, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, M.G.; Bastos, C.E.M.C.; Nagamachi, C.Y.; Pieczarka, J.C.; Vicari, M.R.; Noronha, R.C.R. Physical mapping of repetitive DNA suggests 2n reduction in Amazon turtles Podocnemis (Testudines: Podocnemididae). PLoS ONE 2018, 13, e0197536. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noronha, R.C.R.; Almeida, B.R.R.; Chagas, M.C.S.; Tavares, F.S.; Cardoso, A.L.; Bastos, C.E.M.C.; Silva, N.K.N.; Klautau, A.G.C.M.; Luna, F.O.; Attademo, F.L.N.; et al. Karyotypes of Manatees: New Insights into Hybrid Formation (Trichechus inunguis × Trichechus m. manatus) in the Amazon Estuary. Genes 2022, 13, 1263. https://doi.org/10.3390/genes13071263
Noronha RCR, Almeida BRR, Chagas MCS, Tavares FS, Cardoso AL, Bastos CEMC, Silva NKN, Klautau AGCM, Luna FO, Attademo FLN, et al. Karyotypes of Manatees: New Insights into Hybrid Formation (Trichechus inunguis × Trichechus m. manatus) in the Amazon Estuary. Genes. 2022; 13(7):1263. https://doi.org/10.3390/genes13071263
Chicago/Turabian StyleNoronha, Renata C. R., Bruno R. R. Almeida, Monique C. S. Chagas, Flávia S. Tavares, Adauto L. Cardoso, Carlos E. M. C. Bastos, Natalia K. N. Silva, Alex G. C. M. Klautau, Fábia O. Luna, Fernanda L. N. Attademo, and et al. 2022. "Karyotypes of Manatees: New Insights into Hybrid Formation (Trichechus inunguis × Trichechus m. manatus) in the Amazon Estuary" Genes 13, no. 7: 1263. https://doi.org/10.3390/genes13071263