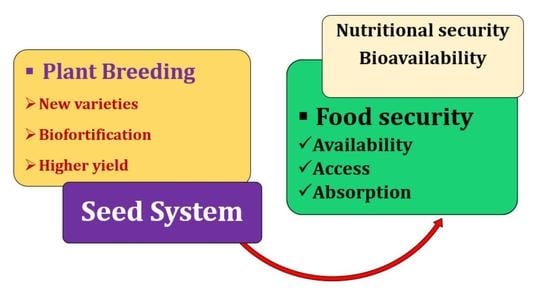
Ensuring Nutritional Security in India through Wheat Biofortification: A Review
Abstract
:1. Introduction
2. Nutritional Security Status of India
3. Biofortification of Wheat
3.1. Acquisition in Plants
3.2. Iron-Zinc Dynamics: Uptake and Translocation
3.3. Bioavailability
4. Genetics and Breeding for Biofortified Wheat
4.1. Quantitative Trait Loci (QTLs) for Zn and Fe
4.2. Marker Trait Associations (MTA)
4.3. Meta-Quantitative Trait Loci (MQTLs)
5. Scaling up Biofortified Wheat Varieties in India
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swaminathan, M.S.; Bhavani, R.V. Food production & availability-essential prerequisites for sustainable food security. Indian J. Med. Res. 2013, 138, 383–391. [Google Scholar] [PubMed] [Green Version]
- Anonymous. World Health Statistics: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Chitekwe, S.; Parajuli, K.R.; Paudyal, N.; Haag, K.C.; Renzaho, A.; Issaka, A.; Agho, K. Individual, household and national factors associated with iron, vitamin A and zinc deficiencies among children aged 6–59 months in Nepal. Matern. Child Nutr. 2021, 18, e13305. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Hossain, M.; Sanin, K.I. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann. Nutr. Metab. 2012, 61, 8–17. [Google Scholar] [CrossRef]
- Grebmer, K.V.; Saltzman, A.; Birol, E.; Wiesmann, D.; Prasai, N.; Yin, S.; Yohannes, Y.; Menon, P.; Thompson, J.; Sonntag, A. Global Hunger Index, the Challenge of Hidden Hunger; IFPRI: Washington, DC, USA, 2014; ISBN 978-0-89629-958-0. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The state of food security and nutrition in the world. In Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Québec City, QC, Canada, 2021. [Google Scholar] [CrossRef]
- Ibba, M.I.; Gupta, O.P.; Govindan, V.; Johnson, A.A.T.; Brinch-Pedersen, H.; Nikolic, M.; Taleon, V. Editorial: Wheat biofortification to alleviate global malnutrition. Front. Nutr. 2022, 9, 1001443. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.P.; Singh, A.K.; Singh, A.; Singh, G.P.; Bansal, K.C.; Datta, S.K. Wheat Biofortification: Utilizing natural genetic diversity, genome-wide association mapping, genomic selection, and genome editing technologies. Front. Nutr. 2022, 12, 826131. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Yadava, D.K.; Hossain, F.; Mohapatra, T. Nutritional security through crop biofortification in India: Status & future prospects. Indian J. Med. Res. 2018, 148, 621–631. [Google Scholar] [CrossRef]
- Saltzman, A.; Birol, E.; Bouis, H.E.; Boy, E.; De Moura, F.F.; Islam, Y.; Pfeiffer, W.H. Biofortification: Progress toward a more nourishing future. Glob. Food Sec. 2013, 2, 9–17. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. Biofortification: Breeding micronutrient-dense crops. Book Breed. Major Food Staples. 2007, 1, 61–91. [Google Scholar] [CrossRef]
- Singh, R.; Velu, G.; Andersson, M.S. Zinc-Biofortified Wheat: Harnessing Genetic Diversity for Improved Nutritional Quality; No. 2187-2019-666; CIMMYT, HarvestPlus and The Global Crop Diversity Trust: Bonn, Germany, 2017. [Google Scholar]
- Prasad, R.S.; Chauhan, J.S.; Sripathy, K.V. An overview of national and international seed quality assurance systems and strategies for energizing seed production chain of field crops in India. Indian J. Agric. Sci. 2017, 87, 287–300. Available online: https://epubs.icar.org.in/index.php/IJAgS/article/view/68592 (accessed on 24 May 2022).
- Krishna, V.V.; Spielman, D.J.; Veettil, C. Exploring the supply and demand factors of varietal turnover in Indian wheat. J. Agric. Sci. 2015, 154, 258–272. [Google Scholar] [CrossRef]
- Kumar, A.; Sankar, R.; Kumar, B.K. Addressing Equity and Social Justice: India’s Transformation of Aspirational Districts Initiative. Available online: https://globalnutritionreport.org/reports/2020-global-nutrition-report (accessed on 26 May 2022).
- Sharma, M.; Kishore, A.; Roy, D.; Joshi, K. A comparison of the Indian diet with the EAT-Lancet reference diet. BMC Public Health 2020, 20, 812. [Google Scholar] [CrossRef]
- Anonymous. Summary Report of EAT Lancet Commission. 2019. Available online: https://eatforum.org/content/uploads/2019/07/EATLancet_Commission_Summary_Report.pdf (accessed on 5 June 2022).
- World Health Organization (WHO). Prevalence of Anaemia in Women of Reproductive Age (Aged 15–49) (%) (who.int) and Prevalence of Anaemia in Children Aged 6–59 Months (%) (who.int). 2022. Available online: https://www.who.int (accessed on 11 October 2022).
- Anonymous. Comprehensive National Nutrition Survey (CNNS) National Report, Ministry of Health and Family Welfare (MoHFW), Government of India, UNICEF and Population Council, New Delhi. 2019. Available online: https://www.unicef.org/india/media/2646/file/CNNS-report.pd (accessed on 22 May 2022).
- Gerard, G.S.; Crespo-Herrera, L.A.; Cross, J.; Mondal, S.; Velu, G.; Juliana, P.; Huerta-Espino, J.; Vargas, M.; Rhandawa, M.S.; Bhavani, S.; et al. Grain yield genetic gains and changes in physiological related traits for CIMMYT’s High Rainfall Wheat Screening Nursery tested across international environments. Field Crop. Res. 2020, 249, 107742. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Morris, C.F. Wheat breeding for quality: A historical review. Cereal Chem. 2018, 95, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Lucht, J.M. Public acceptance of plant biotechnology and GM crops. Viruses 2015, 7, 4254–4281. [Google Scholar] [CrossRef]
- Phougat, D.; Sethi, S.K. Wheat biofortification: Agricultural investment to refrain malnutrition especially in developing world. J. Entomol. Zool. Stud. 2019, 7, 506–511. [Google Scholar]
- Velu, G.; Singh, R.; Huerta-Espino, J.; Pena, J.; Ortiz-Monasterio, I. Breeding for enhanced zinc and iron concentration in CIMMYT spring wheat germplasm. Czech J. Genet. 2011, 47, 174–177. [Google Scholar] [CrossRef] [Green Version]
- Biocropshplus. Available online: https://bcr.harvestplus.org/varieties_released/dashboard (accessed on 11 October 2022).
- Baker, A. Heavy Metals in Soils, 2nd ed.; Alloway, B.J., Ed.; Blackie Academic & Professional: London, UK, 1995; Volume 90, p. 269. ISBN 0-7514-0198-6. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 1995; p. 889. ISBN 9780080571874. [Google Scholar]
- Sarkar, A.N.; Wyn Jones, R.G. Influence of rhizosphere on the nutrient status of dwarf French beans. Plant Soil 1982, 64, 369–380. [Google Scholar] [CrossRef]
- Uygur, V.; Rimmer, D. Reactions of zinc with iron oxide coated calcite surfaces at alkaline pH. Eur. J. Soil Sci. 2000, 51, 511–516. [Google Scholar] [CrossRef]
- Spark, K.M.; Johnson, B.B.; Wells, J.D. Characterizing heavy metal adsorption on iron oxide and oxyhydroxides. Eur. J. Soil Sci. 1995, 46, 621–631. [Google Scholar] [CrossRef]
- Loeppert, R.H.; Hossner, L.R. Reaction of Fe2+ and Fe3+ with calcite. Clays Clay Miner. 1984, 32, 213–222. [Google Scholar] [CrossRef]
- Akhtar, M.; Yousaf, S.; Sarwar, N.; Hussain, S. Zinc biofortification of cereals-role of phosphorus and other impediments in alkaline calcareous soils. Environ. Geochem. Health 2019, 41, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.H.; Yi, H.Y.; Gong, J.M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Ova, E.A.; Kutman, U.B.; Ozturk, L.; Cakmak, I. High phosphorus supply reduced zinc concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant Soil 2015, 393, 147–162. [Google Scholar] [CrossRef]
- Drissi, S.; Houssa, A.A.; Bamouh, A.; Coquant, J.M.; Benbella, M. Effect of zinc-phosphorus interaction on corn silage grown on sandy soil. Agriculture 2015, 5, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, K.; Balakrishnan, N.; Senthil, N. Mycorrhizal symbiosis to increase the grain micronutrient content in maize. Aust. J. Crop Sci. 2013, 7, 900. [Google Scholar]
- Thompson, J.; Clewett, T.; Fiske, M. Field inoculation with arbuscular-mycorrhizal fungi overcomes phosphorus and zinc deficiencies of linseed (Linumusitatissimum) in a vertisol subject to long-fallow disorder. Plant Soil 2013, 371, 117–137. [Google Scholar] [CrossRef]
- Lehmann, A.; Veresoglou, S.D.; Leifheit, E.F.; Rillig, M.C. Arbuscular mycorrhizal influence on zinc nutrition in crop plants-A meta-analysis. Soil Biol. Biochem. 2014, 69, 123–131. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Smith, F.A.; McLaughlin, M.J.; Patti, A.F.; Cavagnaro, T.R. How important is the mycorrhizal pathway for plant Zn uptake? Plant Soil 2015, 390, 157–166. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Bharathi, C.; Jegan, A. Response of maize to mycorrhizal colonization at varying levels of zinc and phosphorus. Biol. Fertil. Soils 2008, 45, 133–144. [Google Scholar] [CrossRef]
- Teng, W.; Deng, Y.; Chen, X.-P.; Xu, X.-F.; Chen, R.-Y.; Lv, Y.; Zhao, Y.-Y.; Zhao, X.-Q.; He, X.; Li, B.; et al. Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J. Exp. Bot. 2013, 64, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, D.; Liu, Y.; Cui, Z.; Chen, X.; Zou, C. Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crop. Res. 2016, 197, 74–82. [Google Scholar] [CrossRef]
- Borrill, P.; Connorton, J.M.; Balk, J.; Miller, A.J.; Sanders, D.; Uauy, C. Biofortification of wheat grain with iron and zinc: Integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 2014, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Graham, R.D.; Ascher, J.S.; Hynes, S.C. Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil 1992, 146, 241–250. [Google Scholar] [CrossRef]
- Sperotto, R.A.; Ricachenevsky, F.K.; Waldow, V.D.; Fett, J.P. Iron Biofortification in rice: It’s a long way to the top. Plant Sci. 2012, 190, 24–39. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Giner-Martínez-Sierra, J.; Orduna, J.; Orera, I.; Rodríguez-Castrillón, J.Á.; García-Alonso, J.I.; Abadía, J.; Álvarez-Fernández, A. Identification of a tri-iron(III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: New insights into plant iron long-distance transport. Plant Cell Physiol. 2010, 51, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Deinlein, U.; Weber, M.; Schmidt, H.; Rensch, S.; Trampczynska, A.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Talke, I.N.; Kräme, U.; et al. Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell. 2012, 24, 708–723. [Google Scholar] [CrossRef] [Green Version]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef] [Green Version]
- Haydon, M.J.; Kawachi, M.; Wirtz, M.; Hillmer, S.; Hell, R.; Krämer, U. Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell. 2012, 24, 724–737. [Google Scholar] [CrossRef]
- Lu, L.; Tian, S.L.; Zhang, J.; Yang, X.; Labavitch, J.M.; Webb, S.M.; Latimer, M.; Brown, P.H. Efficient xylem transport and phloem remobilization of Zn in the hyper accumulator plant species Sedum alfredii. New Phytol. 2013, 198, 721–731. [Google Scholar] [CrossRef]
- Zee, S.Y.; O’Brien, T.P. A special type of tracheary element associated with xylem-discontinuity in floral axis of wheat. Aust. J. Biol. Sci. 1970, 23, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Borg, S.; Brinch-Pedersen, H.; Tauris, B.; Holm, P. Iron transport, deposition and bioavailability in the wheat and barley grain. Plant Soil 2009, 325, 15–24. [Google Scholar] [CrossRef]
- Balmer, Y.; Vensel, W.H.; Dupont, F.M.; Buchanan, B.B.; Hurkman, W.J. Proteome of amyloplasts isolated from developing wheat endosperm presents evidence of broad metabolic capability. J. Exp. Bot. 2006, 57, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Van Lieshout, M.; West, C.E.; Motilal Permaesih, D.; Wang, Y.; Xu, X.; Van Breemen, R.B.; Creemers, A.F.; Verhoeven, M.A.; Lugtenburg, J. Bioefficacy of beta-carotene dissolved in oil studied in children in Indonesia. Am. J. Clin. Nutr. 2001, 73, 949–958. [Google Scholar] [CrossRef] [Green Version]
- La Frano, M.R.; de Moura, F.F.; Erick, B.; Lönnerdal, B.; Burri, B.J. Bioavailability of iron, zinc, and provitamin A carotenoid in biofortified staple crops. Nutr. Rev. 2014, 72, 289–307. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. ICMR-NIN Expert Group on Nutrient Requirement for Indians, Recommended Dietary Allowances (RDA) and Estimated Average Requirement (EAR); ICMR-National Institute of Nutrition: Hyderabad, India, 2020. [Google Scholar]
- Anonymous. National Sample Survey Office. In Nutrition Intake in INDIA, 2011–2012; 560, NSS, 60th Round; NSSO: New Delhi, India, 2014. [Google Scholar]
- Rosado, J.L.; Hambidge, K.M.; Miller, L.V.; Garcia, O.P.; Westcott, J.; Gonzalez, K.; Conde, J.; Hotz, C.; Pfeiffer, W.; Ortiz-Monasterio, I.; et al. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J. Nutr. 2009, 139, 1920–1925. [Google Scholar] [CrossRef] [Green Version]
- House, W.A. Trace element bioavailability as exemplified by iron and zinc. Field Crop. Res. 1999, 60, 115–141. [Google Scholar] [CrossRef]
- Lopez, H.W.; Krespine, V.; Lemaire, A.; Coudray, C.; Coudray, C.F.; Messager, A.; Demigne, C.; Remesy, C. Wheat variety has a major influence on mineral bioavailability studies in rats. J. Cereal Sci. 2003, 37, 257–266. [Google Scholar] [CrossRef]
- Ram, S.; Verma, A.; Sharma, S. Large variability exits in phytase levels among Indian wheat varieties and synthetic hexaploids. J. Cereal Sci. 2010, 52, 486–490. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 200455, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Hambidge, K.M.; Miller, L.V.; Mazariegos, M.; Westcott, J.; Solomons, N.W.; Raboy, V.; Kemp, J.F.; Das, A.; Goco, N.; Hartwell, T.; et al. Upregulation of zinc absorption matches increases in physiologic requirements for zinc in women consuming high or moderate-phytate diets during late pregnancy and early lactation. J. Nutr. 2017, 147, 1079–1085. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Mehta, S.; Udipi, S.A.; Ghugre, P.S.; Luna, S.V.; Wenger, M.J.; Murray-Kolb, L.E.; Przybyszewski, E.M.; Haas, J.D. A Randomized Trial of Iron-Biofortified Pearl Millet in School Children in India. J. Nutr. 2017, 145, 1576–1581. [Google Scholar] [CrossRef] [Green Version]
- Rathan, N.D.; Sehgal, D.; Thiyagarajan, K.; Singh, R.; Singh, A.M.; Velu, G. Identification of Genetic Loci and Candidate Genes Related to Grain Zinc and Iron Concentration Using a Zinc-Enriched Wheat ‘Zinc-Shakti’. Front. Genet. 2021, 12, 652653. [Google Scholar] [CrossRef]
- Xu, Y.; An, D.; Liu, D.; Wang, A.; Xu, H.; Li, B. Molecular mapping of QTLs for grain zinc, iron and protein concentration of wheat across two environments. Field Crop. Res. 2012, 138, 57–62. [Google Scholar] [CrossRef]
- Hao, Y.; Velu, G.; Peña, R.J.; Singh, S.; Singh, R.P. Genetic loci associated with high grain zinc concentration and pleiotropic effect on kernel weight in wheat (Triticum aestivum L.). Mol. Breed. 2014, 34, 1893–1902. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Govindan, V.; Singh, R.P. Quantitative trait loci mapping reveals pleiotropic effect for grain iron and zinc concentrations in wheat. Ann. Appl. Biol. 2016, 169, 27–35. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Govindan, V.; Stangoulis, J.; Hao, Y.; Singh, R.P. QTL mapping of grain zn and fe concentrations in two hexaploid wheat RIL populations with ample transgressive segregation. Front. Plant Sci. 2017, 8, 1800. [Google Scholar] [CrossRef] [Green Version]
- Velu, G.; Singh, R.P.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic dissection of grain zinc concentration in spring wheat for mainstreaming biofortification in CIMMYT wheat breeding. Sci. Rep. 2018, 8, 13526. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, X.; Hao, Y.; Zhang, Y.; Liu, Y.; Pu, Z.; Tian, Y.; Xu, D.; Xia, X.; He, Z.; et al. QTL mapping for grain zinc and iron concentrations in bread wheat. Front. Nutr. 2021, 8, 680391. [Google Scholar] [CrossRef]
- Krishnappa, G.; Rathan, N.D.; Sehgal, D.; Ahlawat, A.K.; Singh, S.K.; Singh, S.K.; Shukla, R.B.; Jaiswal, J.P.; Solanki, I.S.; Singh, G.P.; et al. Identification of Novel Genomic Regions for Biofortification Traits Using an SNP Marker-Enriched Linkage Map in Wheat (Triticum aestivum L.). Front. Nutr. 2021, 8, 669444. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.B.; Rani, S.; Kumar, M.; Gupta, V.; Babu, P.H.; Bainsla, N.K.; Yadav, R. Enhancing the Nutritional Quality of Major Food Crops Through Conventional and Genomics-Assisted Breeding. Front. Nutr. 2020, 7, 533453. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Dreisigacker, S.; Peña, R.J.; Sukumaran, S.; Reynolds, M.P. Genetic characterization of the wheat association mapping initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor. Appl. Genet. 2015, 128, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Cheema, J.; Poland, J.; Uauy, C.; Chhuneja, P. Genome-Wide Association Mapping of grain micronutrients concentration in Aegilops tauschii. Front. Plant Sci. 2019, 10, 54. [Google Scholar] [CrossRef]
- Nozoye, T.; Itai, R.N.; Nagasaka, S.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Diurnal changes in the expression of genes that participate imphytosiderophore synthesis in rice. Soil Sci. Plant Nutr. 2004, 50, 1125–1131. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C.; Chavrier, P. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006, 7, 347–358. [Google Scholar] [CrossRef]
- Singh, S.P.; Jeet, R.; Kumar, J.; Shukla, V.; Srivastava, R.; Mantri, S.S.; Tuli, R. Comparative transcriptional profiling of two wheat genotypes, with contrasting levels of minerals in grains, shows expression differences during grain filling. PLoS ONE 2014, 9, 111718. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-L.; Ogawa, M.; Fleet, C.M.; Zentella, R.; Hu, J.; Heo, J.-O.; Lim, J.; Kamiya, Y.; Yamaguchi, S.; Sun, T.P. Scarecrow-like 3 promotes gibberellin signalling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 2160–2165. [Google Scholar] [CrossRef] [Green Version]
- Cu, S.T.; Guild, G.; Nicolson, A.; Velu, G.; Singh, R.; Stangoulis, J. Genetic dissection of zinc, iron, copper, manganese and phosphorus in wheat (Triticum aestivum L.) grain and rachis at two developmental stages. Plant Sci. 2020, 291, 110338. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Li, T.; Liu, Y.; Yan, Z.; Tang, G.; Zheng, Y.; Liu, D.; Wu, B. Genome-Wide Association Study for grain micronutrient concentrations in wheat advanced lines derived from wild emmer. Front. Plant Sci. 2021, 12, 651283. [Google Scholar] [CrossRef] [PubMed]
- Alomari, D.Z.; Eggert, K.; Von Wirén, N.; Polley, A.; Plieske, J.; Ganal, M.W.; Liu, F.; Pillen, K.; Röder, M.S. Whole-genome association mapping and genomic prediction for iron concentration in wheat grains. Int. J. Mol. Sci. 2018, 20, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatta, M.; Morgounov, A.; Belamkar, V.; Poland, J.; Baenziger, P.S. Unlocking the novel genetic diversity and population structure of synthetic Hexaploid wheat. BMC Genom. 2018, 19, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, J.; Saripalli, G.; Vijay, G.; Neha, G.; Prabina, M.; Prafulla, M.; Deepmala, S.; Sehgal, D.; Vikram, P.; Sansaloni, C.; et al. Genetics of Fe, Zn, β-Carotene, GPC and yield traits in bread wheat (Triticum Aestivum L.) using multi-locus and multi-traits GWAS. Euphytica 2018, 214, 1–17. [Google Scholar] [CrossRef]
- Gorafi, Y.S.A.; Kim, J.S.; Elbashir, A.A.E.; Tsujimoto, H. A population of wheat multiple synthetic derivatives: An effective platform to explore, harness and utilize genetic diversity of Aegilops tauschii for wheat improvement. Theor. Appl. Genet. 2018, 131, 1615–1626. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B.; Tahmasebi, A.; Richards, C. Comparative genomic analysis of quantitative trait loci associated with micronutrient contents, grain quality, and agronomic traits in wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 709817. [Google Scholar] [CrossRef]
- Leung, H.; Raghavan, C.; Zhou, B.; Oliva, R.; Choi, I.R.; Lacorte, V.; Casiana, M.L.; Cruz, V.; Gregorio, G.; Singh, R.K.; et al. Allele mining and enhanced genetic recombination for rice breeding. Rice 2015, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, D.; Yamamoto, E.; Ohtani, T.; Kanno, N.; Tsunematsu, H.; Nonoue, Y.; Yano, M.; Yamamoto, T.; Yonemaru, J.-I. Haplotype-based allele mining in the Japan-MAGIC rice population. Sci. Rep. 2018, 8, 4379. [Google Scholar] [CrossRef] [Green Version]
- Virk, P.S.; Andersson, M.S.; Arcos, J.; Govindaraj, M.; Pfeiffer, W.H. Transition from targeted breeding to mainstreaming of biofortification traits in crop improvement programs. Front. Plant Sci. 2021, 14, 703990. [Google Scholar] [CrossRef]
- Seednet India Portal. National Initiative for Information on Quality Seed, Ministry of Agriculture and Farmers Welfare, Govt. of India. 2021. Available online: https://seednet.gov.in/ (accessed on 4 March 2022).
- Anonymous. Pathway for Addressing Malnutrition in India Farming System for Nutrition. In Policy Recommendations; MS Swaminathan Research Foundation: Chennai, India, 2018; pp. 1–32. Available online: http://59.160.153.188/library/sites/default/files/Policy%20Recommendations%20Final%2027%20Mar%2019.pdf (accessed on 9 September 2022).
- Talsma, E.F.; Melse-Boonstra, A.; Brouwer, I.D. Acceptance and adoption of biofortified crops in low-and middle-income countries: A systematic review. Nutr. Rev. 2017, 75, 798–829. [Google Scholar] [CrossRef]
- Nagarajan, S.; Bhavani, R.V.; Swaminathan, M.S. Operationalizing the concept of farming system for nutrition through the promotion of nutrition-sensitive agriculture. Curr. Sci. 2014, 107, 959–964. [Google Scholar]



| Nutrients | Baseline Level | Level Achieved by Biofortification | Target |
|---|---|---|---|
| Protein | 8–10 (%) | More than 12.0 (%) | 12–15 (%) |
| Iron | 28–32 ppm | More than 38 ppm | 40–50 ppm |
| Zinc | 30–32 ppm | More than 40 ppm | 40–50 ppm |
| S. No. | Pedigree | No. of QTLs | Ref. |
|---|---|---|---|
| Cross Type (Number) | Zn Fe | ||
| 1. | Kachu/Zinc-Shakti RILs (190) | 09 04 | [67] |
| 2. | Jingdong 8//Bainong AK58 RILs (254) | 07 04 | [73] |
| 3. | WH542/synthetic derivative (Triticum dicoccon PI94624/Aegilops tauschii RILs 163) (409)//BCN) | 06 03 | [74] |
| 4 | No. of identified QTLs since 2009–2019 in different mapping populations | 111 93 | [75] |
| Total no. of identified QTLs | 133 104 |
| S. N. | Association Panel (Size) | Location(s) | Environments | Method for Estimation | Markers | MTAs | Ref. |
|---|---|---|---|---|---|---|---|
| Zn Fe | |||||||
| 1 | HPAM Panel (330) | India, Mexico | 6 | EDXRF | 14,273 | 39 - | [72] |
| 2 | Chinese winter wheat grain (205) | China | 4 | ICP-MS | 24,355 | 3 5 | [73] |
| 3 | Ae. Tauschii panel (114) | India | 3 | ICP-OES | 5249 | 4 5 | [78] |
| 4 | HPAM Panel (330) | Mexico | 2 | ICP-MS | 28,074 | 72 65 | [83] |
| 5 | HPAM panel (330) | Mexico | 3 | ICP-MS 7500x | 28,074 | 5 -- | [83] |
| 6 | CN16 x D1-Wild emmer wheat-advanced lines (161) | China | 4 | PinAAcle 900T, USA | 13,116 | 3 6 | [84] |
| 7 | EuWV Panel (369) Sub-panel (183) | Germany | 3 3 | ICP-OES ICP-OES | 15,523 28,710 (Zn) 44,233 (Fe) | 40 41 161 - - 137 | [85] |
| 8 | SHW (123) | Turkey | 2 | ICP-MS | 35,648 | 13 03 | [86] |
| 9 | SWRS (246) | India | 2 | EDXRF | - | 94 33 | [87] |
| 10 | SHW (Longdon × 47 Ae tauschii) (47) | Japan | 2 | ICP-AES | 70 (SSRs) | 03 03 | [88] |
| Chromosome No. | A-Genome | B-Genome | D-Genome |
|---|---|---|---|
| 1 | T.monococcum T.aestivum | T.aestivum, T. durum | T. aestivum |
| 2 | T. durum, T. dicoccoides | T.aestivum | - |
| 3 | T.aestivum | - | T. aestivum |
| 4 | T. aestivum | T. aestivum | T. aestivum |
| 5 | T. aestivum, T. dicoccoides, T. monococcum | T.aestivum | - |
| 6 | T. spelta, T. aestivum | T. aestivum T. dicoccoides, T. durum | - |
| 7 | T. aestivum, T. dicoccoides, T. boeoticum, T. monococcum | T. dicoccoides | - |
| S.No. | Variety | Growing Conditions | Quality Trait | Year of Notification | Breeder Seed (Quintals) | |
|---|---|---|---|---|---|---|
| Indent | Production | |||||
| 1 | DBW 187 | NWPZ (IR-ES and TS) and NEPZ (IR-TS) | Fe (41.3ppm) and Zn (43.7 ppm) | 2020 | 1617.35 | 2315.00 |
| 2 | DDW 47 (d) | CZ (RI-TS) | Fe (40.1 ppm) | 2020 | 155.00 | 269.10 |
| 3 | PBW 771 | NWPZ (IR-LS) | Zn (41.4 ppm) | 2020 | 71.80 | 80.00 |
| 4 | HD 3249 | NEPZ (IR-TS) | Fe (42.5 ppm) | 2020 | 35.80 | 40.00 |
| 5 | PBW 752 | NWPZ (IR-LS) | Protein (12.4%) | 2019 | 71.80 | 77.00 |
| 6 | PBW-757 | NWPZ (IR-VLS) | Zn (42.3 pm) | 2019 | 33.00 | 33.00 |
| 7 | HI-8777 (d) | CZ (RF-TS) | Fe (48.7 ppm) and Zn (43.6 ppm) | 2018 | 2.00 | 110.50 |
| 8 | DBW 173 | NWPZ (IR-LS) | Protein (12.5%) and Fe (40.7 ppm) | 2018 | 170.00 | 198.00 |
| 9 | MACS 4028 | PZ (IR-TS) | Protein (14.2%), Fe (46.1 ppm) and Zn (40.3 ppm) | 2018 | 2.00 | 5.00 |
| 10 | UAS-375 | PZ (RI-TS) | Protein (13.8%) | 2018 | 2.00 | 3.00 |
| 11 | PBW 1 ZN | NWPZ (IR-TS) | Fe (40 ppm) and Zn (40.6 ppm) | 2017 | 191.00 | 240.00 |
| 12 | WB-2 | NWPZ and Bihar (IR-TS) | Zn (42 ppm) and Fe (40 ppm) | 2017 | 80.20 | 90.00 |
| 13 | HD-3171 | NEPZ (RI-TS) | Fe (47.1 ppm) | 2017 | 56.45 | 10.00 |
| 14 | HI 8759 | CZ(IR-TS) | Fe (41.1 ppm) and Zn (42.8 ppm) | 2017 | 846.20 | 420.00 |
| Total | 3334.60 | 3890.60 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamble, U.; Mishra, C.N.; Govindan, V.; Sharma, A.K.; Pawar, S.; Kumar, S.; Krishnappa, G.; Gupta, O.P.; Singh, G.P.; Singh, G. Ensuring Nutritional Security in India through Wheat Biofortification: A Review. Genes 2022, 13, 2298. https://doi.org/10.3390/genes13122298
Kamble U, Mishra CN, Govindan V, Sharma AK, Pawar S, Kumar S, Krishnappa G, Gupta OP, Singh GP, Singh G. Ensuring Nutritional Security in India through Wheat Biofortification: A Review. Genes. 2022; 13(12):2298. https://doi.org/10.3390/genes13122298
Chicago/Turabian StyleKamble, Umesh, Chandra Nath Mishra, Velu Govindan, Amit Kumar Sharma, Sushma Pawar, Satish Kumar, Gopalareddy Krishnappa, Om Prakash Gupta, Gyanendra Pratap Singh, and Gyanendra Singh. 2022. "Ensuring Nutritional Security in India through Wheat Biofortification: A Review" Genes 13, no. 12: 2298. https://doi.org/10.3390/genes13122298