Human Amnion Epithelial Cells Impair T Cell Proliferation: The Role of HLA-G and HLA-E Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Preparations
2.2. Cell Culture
2.3. Isolation of EV from hAEC
2.4. Inhibition of EV Release
2.5. Flow Cytometry
2.6. ELISA
2.7. Cell Proliferation Assay
2.8. Gene Profiling by qPCR
2.9. Statistical Analysis
3. Results
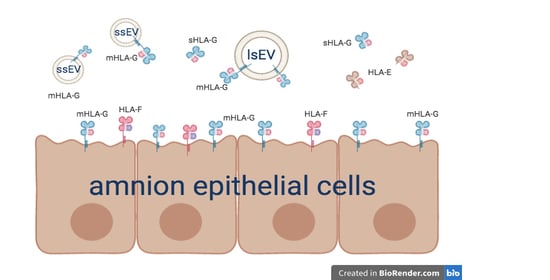
3.1. Human Amnion Epithelial Cells Express and Release HLA-Ib Molecules
3.2. hAEC-Derived EV Expressed HLA-G and Epithelial Markers
3.3. HLA-G is Involved in hAEC-Mediated Inhibition of Allogeneic T Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gramignoli, R.; Srinivasan, R.C.; Kannisto, K.; Strom, S.C. Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures. Curr. Protoc. Stem. Cell Biol. 2016, 37, 1E.10.1–1E.10.13. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [Green Version]
- Bryzek, A.; Czekaj, P.; Plewka, D.; Komarska, H.; Tomsia, M.; Lesiak, M.; Sieron, A.L.; Sikora, J.; Kopaczka, K. Expression and co-expression of surface markers of pluripotency on human amniotic cells cultured in different growth media. Ginekol. Pol. 2013, 84, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Gramignoli, R. Therapeutic Use of Human Amnion-Derived Products: Cell-Based Therapy for Liver Disease. Curr. Pathobiol. Rep. 2016, 4, 157–167. [Google Scholar] [CrossRef]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [Green Version]
- Marongiu, F.; Gramignoli, R.; Dorko, K.; Miki, T.; Ranade, A.R.; Paola Serra, M.; Doratiotto, S.; Sini, M.; Sharma, S.; Mitamura, K.; et al. Hepatic differentiation of amniotic epithelial cells. Hepatology 2011, 53, 1719–1729. [Google Scholar] [CrossRef] [Green Version]
- Skvorak, K.J.; Dorko, K.; Marongiu, F.; Tahan, V.; Hansel, M.C.; Gramignoli, R.; Arning, E.; Bottiglieri, T.; Gibson, K.M.; Strom, S.C. Improved amino acid, bioenergetic metabolite and neurotransmitter profiles following human amnion epithelial cell transplant in intermediate maple syrup urine disease mice. Mol. Genet. Metab. 2013, 109, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Skvorak, K.J.; Dorko, K.; Marongiu, F.; Tahan, V.; Hansel, M.C.; Gramignoli, R.; Gibson, K.M.; Strom, S.C. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology 2013, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strom, S.C.; Skvorak, K.; Gramignoli, R.; Marongiu, F.; Miki, T. Translation of amnion stem cells to the clinic. Stem Cells Dev. 2013, 22 (Suppl. 1), 96–102. [Google Scholar] [CrossRef]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants With Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Lim, R.; Mockler, J.C.; Wallace, E.M. Two-year outcomes of infants enrolled in the first-in-human study of amnion cells for bronchopulmonary dysplasia. Stem Cells Transl. Med. 2020, 9, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strom, S.C.; Gramignoli, R. Human amnion epithelial cells expressing HLA-G as novel cell-based treatment for liver disease. Hum. Immunol. 2016, 77, 734–739. [Google Scholar] [CrossRef]
- Banas, R.A.; Trumpower, C.; Bentlejewski, C.; Marshall, V.; Sing, G.; Zeevi, A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum. Immunol. 2008, 69, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in culture expanded human amniotic epithelial cells: Implications for potential therapeutic applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tee, J.Y.; Vaghjiani, V.; Liu, Y.H.; Murthi, P.; Chan, J.; Manuelpillai, U. Immunogenicity and immunomodulatory properties of hepatocyte-like cells derived from human amniotic epithelial cells. Curr. Stem Cell Res. Ther. 2013, 8, 91–99. [Google Scholar] [PubMed]
- Rizzo, R.; Vercammen, M.; van de Velde, H.; Horn, P.A.; Rebmann, V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell. Mol. Life Sci. 2011, 68, 341–352. [Google Scholar] [CrossRef]
- Le Bouteiller, P.; Lenfant, F. Antigen-presenting function(s) of the non-classical HLA-E, -F and -G class I molecules: The beginning of a story. Res. Immunol. 1996, 147, 301–313. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; McMichael, A.J. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr. Opin. Immunol. 1999, 11, 100–108. [Google Scholar] [CrossRef]
- Mallet, V.; Fournel, S.; Schmitt, C.; Campan, A.; Lenfant, F.; Le Bouteiller, P. Primary cultured human thymic epithelial cells express both membrane-bound and soluble HLA-G translated products. J. Reprod. Immunol. 1999, 43, 225–234. [Google Scholar] [CrossRef]
- Crisa, L.; McMaster, M.T.; Ishii, J.K.; Fisher, S.J.; Salomon, D.R. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J. Exp. Med. 1997, 186, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Higa, K.; Shimmura, S.; Shimazaki, J.; Tsubota, K. Ocular surface epithelial cells up-regulate HLA-G when expanded in vitro on amniotic membrane substrates. Cornea 2006, 25, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.J.; Huang, L.; Leung, T.Y.; Burd, A. A study of the immune properties of human umbilical cord lining epithelial cells. Cytotherapy 2014, 16, 631–639. [Google Scholar] [CrossRef]
- Kolanko, E.; Kopaczka, K.; Koryciak-Komarska, H.; Czech, E.; Szmytkowska, P.; Gramignoli, R.; Czekaj, P. Increased immunomodulatory capacity of human amniotic cells after activation by pro-inflammatory chemokines. Eur. J. Pharmacol. 2019, 859, 172545. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; De Munari, S.; Vertua, E.; Gibelli, L.; Wengler, G.S.; Parolini, O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells 2008, 26, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wolbank, S.; Peterbauer, A.; Fahrner, M.; Hennerbichler, S.; van Griensven, M.; Stadler, G.; Redl, H.; Gabriel, C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007, 13, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- LeBouder, F.; Khoufache, K.; Menier, C.; Mandouri, Y.; Keffous, M.; Lejal, N.; Krawice-Radanne, I.; Carosella, E.D.; Rouas-Freiss, N.; Riteau, B. Immunosuppressive HLA-G molecule is upregulated in alveolar epithelial cells after influenza A virus infection. Hum. Immunol. 2009, 70, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Loisel, D.A.; Stern, R.; Laxman, B.; Floreth, T.; Marroquin, B.A. Human leukocyte antigen-G expression in differentiated human airway epithelial cells: Lack of modulation by Th2-associated cytokines. Respir. Res. 2013, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Pistoia, V.; Morandi, F.; Wang, X.; Ferrone, S. Soluble HLA-G: Are they clinically relevant? Semin. Cancer Biol. 2007, 17, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Carosella, E.D.; Rouas-Freiss, N.; Roux, D.T.-L.; Moreau, P.; LeMaoult, J. Chapter Two-HLA-G: An Immune Checkpoint Molecule. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 127, pp. 33–144. [Google Scholar]
- Zilberman, S.; Schenowitz, C.; Agaugué, S.; Benoît, F.; Riteau, B.; Rouzier, R.; Carosella, E.D.; Rouas-Freiss, N.; Menier, C. HLA-G1 and HLA-G5 active dimers are present in malignant cells and effusions: The influence of the tumor microenvironment. Eur. J. Immunol. 2012, 42, 1599–1608. [Google Scholar] [CrossRef] [Green Version]
- Braud, V.M.; Allan, D.S.; Wilson, D.; McMichael, A.J. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr. Biol. 1998, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Söderström, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Yan, W.H. The Emerging Roles of Human Leukocyte Antigen-F in Immune Modulation and Viral Infection. Front. Immunol. 2019, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Alhomrani, M.; Correia, J.; Zavou, M.; Leaw, B.; Kuk, N.; Xu, R.; Saad, M.I.; Hodge, A.; Greening, D.W.; Lim, R.; et al. The Human Amnion Epithelial Cell Secretome Decreases Hepatic Fibrosis in Mice with Chronic Liver Fibrosis. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Tan, J.L.; Lau, S.N.; Leaw, B.; Nguyen, H.P.T.; Salamonsen, L.A.; Saad, M.I.; Chan, S.T.; Zhu, D.; Krause, M.; Kim, C.; et al. Amnion Epithelial Cell-Derived Exosomes Restrict Lung Injury and Enhance Endogenous Lung Repair. Stem Cells Transl. Med. 2018, 7, 180–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 2017, 31, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Ohga, N.; Akiyama, K.; Hirata, N.; Kitahara, S.; Maishi, N.; Osawa, T.; Yamamoto, K.; Kondoh, M.; Shindoh, M.; et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS ONE 2012, 7, e34045. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Marimpietri, D.; Horenstein, A.L.; Bolzoni, M.; Toscani, D.; Costa, F.; Castella, B.; Faini, A.C.; Massaia, M.; Pistoia, V.; et al. Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD+. Oncoimmunology 2018, 7, e1458809. [Google Scholar] [CrossRef] [Green Version]
- Morandi, F.; Marimpietri, D.; Horenstein, A.L.; Corrias, M.V.; Malavasi, F. Microvesicles expressing adenosinergic ectoenzymes and their potential role in modulating bone marrow infiltration by neuroblastoma cells. Oncoimmunology 2019, 8, e1574198. [Google Scholar] [CrossRef] [Green Version]
- Rebmann, V.; König, L.; Nardi, F.d.S.; Wagner, B.; Manvailer, L.F.S.; Horn, P.A. The Potential of HLA-G-Bearing Extracellular Vesicles as a Future Element in HLA-G Immune Biology. Front. Immunol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, E.; Tripodo, C.; Pagnan, G.; Guarnotta, C.; Marimpietri, D.; Corrias, M.V.; Ribatti, D.; Zupo, S.; Fraternali-Orcioni, G.; Ravetti, J.L.; et al. The interleukin (IL)-31/IL-31R axis contributes to tumor growth in human follicular lymphoma. Leukemia 2015, 29, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Marimpietri, D.; Petretto, A.; Raffaghello, L.; Pezzolo, A.; Gagliani, C.; Tacchetti, C.; Mauri, P.; Melioli, G.; Pistoia, V. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS ONE 2013, 8, e75054. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Mäger, I.; Lee, Y.; Görgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E.L. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Bostancioglu, R.B.; Welsh, J.A.; Zickler, A.M.; Murke, F.; Corso, G.; Felldin, U.; Hagey, D.W.; Evertsson, B.; Liang, X.M.; et al. Systematic Methodological Evaluation of a Multiplex Bead-Based Flow Cytometry Assay for Detection of Extracellular Vesicle Surface Signatures. Front. Immunol. 2018, 9, 1326. [Google Scholar] [CrossRef] [Green Version]
- Morandi, F.; Venturi, C.; Rizzo, R.; Castellazzi, M.; Baldi, E.; Caniatti, M.L.; Tola, M.R.; Granieri, E.; Fainardi, E.; Uccelli, A.; et al. Intrathecal soluble HLA-E correlates with disease activity in patients with multiple sclerosis and may cooperate with soluble HLA-G in the resolution of neuroinflammation. J. Neuroimmune Pharmacol. 2013, 8, 944–955. [Google Scholar] [CrossRef]
- Gramignoli, R.; Vosough, M.; Kannisto, K.; Srinivasan, R.C.; Strom, S.C. Clinical hepatocyte transplantation: Practical limits and possible solutions. Eur. Surg. Res. 2015, 54, 162–177. [Google Scholar] [CrossRef]
- Grinyó, J.M.; Cruzado, J.M. Mycophenolate mofetil and calcineurin-inhibitor reduction: Recent progress. Am. J. Transpl. 2009, 9, 2447–2452. [Google Scholar] [CrossRef]
- Akle, C.A.; Adinolfi, M.; Welsh, K.I.; Leibowitz, S.; McColl, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981, 2, 1003–1005. [Google Scholar] [CrossRef]
- Bembi, B.; Comelli, M.; Scaggiante, B.; Pineschi, A.; Rapelli, S.; Gornati, R.; Montorfano, G.; Berra, B.; Agosti, E.; Romeo, D. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am. J. Med. Genet. 1992, 44, 527–533. [Google Scholar] [CrossRef]
- Yeager, A.M.; Singer, H.S.; Buck, J.R.; Matalon, R.; Brennan, S.; O’Toole, S.O.; Moser, H.W. A therapeutic trial of amniotic epithelial cell implantation in patients with lysosomal storage diseases. Am. J. Med. Genet. 1985, 22, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Chan, J.; Vaghjiani, V.; Murthi, P.; Manuelpillai, U.; Toh, B.H. Human amniotic epithelial cells suppress relapse of corticosteroid-remitted experimental autoimmune disease. Cytotherapy 2014, 16, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Vaghjiani, V.; Tee, J.Y.; To, K.; Cui, P.; Oh, D.Y.; Manuelpillai, U.; Toh, B.H.; Chan, J. Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS ONE 2012, 7, e35758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morandi, F.; Horenstein, A.L.; Quarona, V.; Faini, A.C.; Castella, B.; Srinivasan, R.C.; Strom, S.C.; Malavasi, F.; Gramignoli, R. Ectonucleotidase Expression on Human Amnion Epithelial Cells: Adenosinergic Pathways and Dichotomic Effects on Immune Effector Cell Populations. J. Immunol. 2019, 202, 724–735. [Google Scholar] [CrossRef] [Green Version]
- McDonald, C.A.; Payne, N.L.; Sun, G.; Moussa, L.; Siatskas, C.; Lim, R.; Wallace, E.M.; Jenkin, G.; Bernard, C.C. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2015, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Coupel, S.; Moreau, A.; Hamidou, M.; Horejsi, V.; Soulillou, J.P.; Charreau, B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood 2007, 109, 2806–2814. [Google Scholar] [CrossRef] [Green Version]
- Derre, L.; Corvaisier, M.; Charreau, B.; Moreau, A.; Godefroy, E.; Moreau-Aubry, A.; Jotereau, F.; Gervois, N. Expression and release of HLA-E by melanoma cells and melanocytes: Potential impact on the response of cytotoxic effector cells. J. Immunol. 2006, 177, 3100–3107. [Google Scholar] [CrossRef] [Green Version]
- Gavlovsky, P.J.; Tonnerre, P.; Guitton, C.; Charreau, B. Expression of MHC class I-related molecules MICA, HLA-E and EPCR shape endothelial cells with unique functions in innate and adaptive immunity. Hum. Immunol. 2016, 77, 1084–1091. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morandi, F.; Marimpietri, D.; Görgens, A.; Gallo, A.; Srinivasan, R.C.; El-Andaloussi, S.; Gramignoli, R. Human Amnion Epithelial Cells Impair T Cell Proliferation: The Role of HLA-G and HLA-E Molecules. Cells 2020, 9, 2123. https://doi.org/10.3390/cells9092123
Morandi F, Marimpietri D, Görgens A, Gallo A, Srinivasan RC, El-Andaloussi S, Gramignoli R. Human Amnion Epithelial Cells Impair T Cell Proliferation: The Role of HLA-G and HLA-E Molecules. Cells. 2020; 9(9):2123. https://doi.org/10.3390/cells9092123
Chicago/Turabian StyleMorandi, Fabio, Danilo Marimpietri, Andre Görgens, Alessia Gallo, Raghuraman Chittor Srinivasan, Samir El-Andaloussi, and Roberto Gramignoli. 2020. "Human Amnion Epithelial Cells Impair T Cell Proliferation: The Role of HLA-G and HLA-E Molecules" Cells 9, no. 9: 2123. https://doi.org/10.3390/cells9092123