Anti-HLA Class II Antibodies Correlate with C-Reactive Protein Levels in Patients with Rheumatoid Arthritis Associated with Interstitial Lung Disease
Abstract
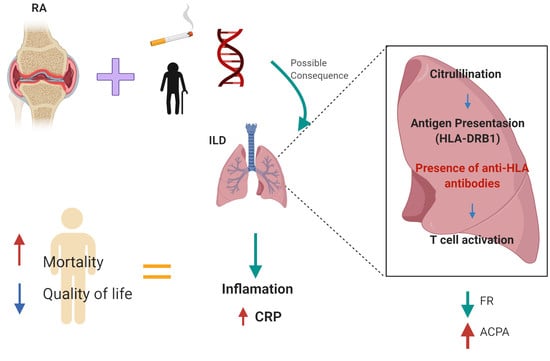
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics Statement
2.3. Anti-HLA Antibodies Detection
2.4. Statistical Analysis
3. Results
3.1. Clinical features of the ILD patients
3.2. Panel-Reactive Antibodies (PRA) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klareskog, L.; Catrina, A.I.; Paget, S. Rheumatoid arthritis. Lancet 2009, 373, 1–14. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Michaud, K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 2009, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Doyle, T.J.; Dellaripa, P.F. Lung Manifestations in the Rheumatic Diseases. Chest 2017, 152, 1283–1295. [Google Scholar] [CrossRef]
- Richman, N.C.; Yazdany, J.; Graf, J.; Chernitskiy, V.; Imboden, J.B. Extraarticular Manifestations of Rheumatoid Arthritis in a Multiethnic Cohort of Predominantly Hispanic and Asian Patients. Medicine (Baltimore) 2013, 92, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Hyldgaard, C.; Hilberg, O.; Pedersen, A.B.; Ulrichsen, S.P.; Løkke, A.; Bendstrup, E.; Ellingsen, T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann. Rheum. Dis. 2017, 76, 1700–1706. [Google Scholar] [CrossRef]
- Kim, D.; Cho, S.-K.; Choi, C.-B.; Choe, J.-Y.; Chung, W.T.; Hong, S.-J.; Jun, J.-B.; Jung, Y.O.; Kim, T.-H.; Kim, T.-J.; et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol. Int. 2017, 37, 1735–1745. [Google Scholar] [CrossRef]
- Busch, R. HLA associations in inflammatory arthritis: Emerging mechanisms and clinical implications. Nat. Rev. Rheumatol. 2019, 6, 364–381. [Google Scholar] [CrossRef] [Green Version]
- Horton, R.; Wilming, L.; Rand, V.; Lovering, R.C.; Bruford, E.; Khodiyar, V.; Lush, M.J.; Povey, S.; Talbot, C.C.; Wright, M.; et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004, 5, 889–899. [Google Scholar] [CrossRef]
- Dieude, P.; Cornélis, F. Genetic basis of rheumatoid arthritis. Jt. Bone Spine 2005, 72, 520–526. [Google Scholar] [CrossRef]
- Gough, S.C.L.; Simmonds, M.J. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr. Genom. 2007, 8, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt, H.; Sehnert, B.; Bockermann, R.; Engström, Å.; Kalden, J.R.; Holmdahl, R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur. J. Immunol. 2005, 35, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Akgul, S.; Ciftci, H.; Temurhan, S.; Caliskan, Y.; Bayraktar, A.; Tefik, T.; Kaya, I.; Canitez, I.; Demir, E.; Yazici, H.; et al. Association Between HLA Antibodies and Different Sensitization Events in Renal Transplant Candidates. Transplant. Proc. 2017, 49, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Triulzi, D.; Kleinman, S.; Kakaiya, R.M.; Busch, M.P.; Norris, P.J.; Steele, W.R.; Glynn, S.A.; Hillyer, C.D.; Carey, P.; Gottschall, J.L.; et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: Implications for a transfusion-related acute lung injury risk reduction strategy. Transfus. 2009, 49, 1825–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Dankers, M.K.; Roelen, D.L.; Korfage, N.; De Lange, P.; Witvliet, M.; Sandkuijl, L.; Doxiadis, I.I.N.; Claas, F. Differential immunogenicity of paternal HLA Class I antigens in pregnant women. Hum. Immunol. 2003, 64, 600–606. [Google Scholar] [CrossRef]
- Geneugelijk, K.; Hönger, G.; Van Deutekom, H.W.M.; Hösli, I.M.; Schaub, S.; Spierings, E. A Previous Miscarriage and a Previous Successful Pregnancy Have a Different Impact on HLA Antibody Formation during a Subsequent Successful Pregnancy. Front. Immunol. 2016, 7, 1453. [Google Scholar] [CrossRef] [Green Version]
- Gammill, H.S.; Nelson, J.L. Naturally acquired microchimerism. Int. J. Dev. Boil. 2010, 54, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Artlett, C.M.; Cox, L.A.; Ramos, R.C.; Dennis, T.N.; Fortunato, R.A.; Hummers, L.; Jimenez, S.A.; Smith, J.B. Increased Microchimeric CD4+ T Lymphocytes in Peripheral Blood from Women with Systemic Sclerosis. Clin. Immunol. 2002, 103, 303–308. [Google Scholar] [CrossRef]
- Sarkar, K.; Miller, F. Possible roles and determinants of microchimerism in autoimmune and other disorders. Autoimmun. Rev. 2004, 3, 454–463. [Google Scholar] [CrossRef]
- Crowson, C.S.; Matteson, E.L.; Myasoedova, E.; Michet, C.J.; Ernste, F.C.; Warrington, K.J.; Davis, J.M.; Hunder, G.G.; Therneau, T.M.; Gabriel, S.E. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011, 63, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Rak, J.M.; Maestroni, L.; Balandraud, N.; Guis, S.; Boudinet, H.; Guzian, M.C.; Yan, Z.; Azzouz, D.; Auger, I.; Roudier, C.; et al. Transfer of the shared epitope through microchimerism in women with rheumatoid arthritis. Arthritis Rheum. 2009, 60, 73–80. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Liang, C.; Li, J.; Lu, C.; Xie, D.; Liu, J.; Zhong, C.; Wu, X.; Dai, R.; Zhang, H.; Guan, D.; et al. HIF1α inhibition facilitates Leflunomide-AHR-CRP signaling to attenuate bone erosion in CRP-aberrant rheumatoid arthritis. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Plant, M.J.; Williams, A.L.; O’Sullivan, M.M.; Lewis, P.A.; Coles, E.C.; Jessop, J.D. Relationship between time-integrated C-reactive protein levels and radiologic progression in patients with rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1473–1477. [Google Scholar] [CrossRef]
- Kim, K.; Jiang, X.; Cui, J.; Lu, B.; Costenbader, K.H.; Sparks, J.A.; Bang, S.-Y.; Lee, H.-S.; Okada, Y.; Raychaudhuri, S.; et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 2611–2623. [Google Scholar] [CrossRef]
- Anderson, J.; Caplan, L.; Yazdany, J.; Robbins, M.L.; Neogi, T.; Michaud, K.; Saag, K.G.; O’Dell, J.R.; Kazi, S. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Rheum. 2012, 64, 640–647. [Google Scholar] [CrossRef]
- International, S.; Consensus, M. American Thoracic Society/ European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society. Am. Thorac Soc. 2002, 165. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Smolen, J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): A review of their usefulness and validity in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, S100–S108. [Google Scholar] [CrossRef]
- Colombo, M.B.; Haworth, S.E.; Poli, F.; Nocco, A.; Puglisi, G.; Innocente, A.; Serafini, M.; Messa, P.; Scalamogna, M. Luminex technology for anti-HLA antibody screening: Evaluation of performance and of impact on laboratory routine. Cytom. Part. B Clin. Cytom. 2007, 72, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Lopes, L.; Chiba, A.K.; Ruiz, M.O.; Bordin, J.O.; Fabron-Jr, A. Impact of using different laboratory assays to detect human leukocyte antigen antibodies in female blood donors. Transfusion 2010, 50, 902–908. [Google Scholar] [CrossRef]
- One Lambda, I. HLA FusionTM Software 4.x.x User Manual. 2012. Available online: https://manualzz.com/doc/6750498/hla-fusiontm-ivd-and-research-software-v4.xx (accessed on 11 March 2020).
- RStudio. Available online: https://rstudio.com/ (accessed on 27 December 2019).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/epiinfo/index.html (accessed on 27 December 2019).
- Koduri, G.; Norton, S.; Young, A.; Cox, N.; Davies, P.; Devlin, J.; Dixey, J.; Gough, A.; Prouse, P.; Winfield, J.; et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: Results from an inception cohort. Rheumatology 2010, 49, 1483–1489. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Legoff, J.A.; Krause, M.L.; Crowson, C.S.; Ryu, J.; Matteson, E.L. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology 2016, 56, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Shi, Y.; Wang, X.; Huang, H.; Ascherman, D.P. Asymptomatic Preclinical Rheumatoid Arthritis-Associated Interstitial Lung Disease. Clin. Dev. Immunol. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.-Y.; Lee, K.-H.; Cho, S.-K.; Lee, H.-S.; Lee, K.W.; Bae, S.-C. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of RF and ACPA. Arthritis Rheum. 2010, 62, 369–377. [Google Scholar] [CrossRef]
- Mori, S.; Koga, Y.; Sugimoto, M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir. Med. 2012, 106, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Makrygiannakis, D.; Hermansson, M.; Ulfgren, A.-K.; Nicholas, A.P.; Zendman, A.J.W.; Grunewald, J.; Klareskog, L.; Catrina, A.I.; Eklund, A.; Skold, C.M. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 2008, 67, 1488–1492. [Google Scholar] [CrossRef]
- Solomon, J.J.; Ryu, J.; Tazelaar, H.D.; Myers, J.L.; Tuder, R.M.; Cool, C.D.; Curran-Everett, D.; Fischer, A.; Swigris, J.J.; Brown, K.K. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir. Med. 2013, 107, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, H.; Wu, N.; Dong, X.; Zheng, Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 2017, 36, 817–823. [Google Scholar] [CrossRef]
- Schellekens, G.A.; Visser, H.; De Jong, B.A.W.; van den Hoogen, F.H.; Hazes, J.M.; Breedveld, F.C.; van Venrooij, W.J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000, 43, 155–163. [Google Scholar] [CrossRef]
- Aubart, F.; Crestani, B.; Nicaise-Roland, P.; Tubach, F.; Bollet, C.; Dawidowicz, K.; Quintin, E.; Hayem, G.; Palazzo, E.; Meyer, O.; et al. High Levels of Anti-Cyclic Citrullinated Peptide Autoantibodies Are Associated with Co-occurrence of Pulmonary Diseases with Rheumatoid Arthritis. J. Rheumatol. 2011, 38, 979–982. [Google Scholar] [CrossRef]
- Yin, Y.; Liang, D.; Zhao, L.; Li, Y.; Liu, W.; Ren, Y.; Li, Y.; Zeng, X.; Zhang, F.; Tang, F.; et al. Anti-Cyclic Citrullinated Peptide Antibody Is Associated with Interstitial Lung Disease in Patients with Rheumatoid Arthritis. PLoS ONE 2014, 9, e92449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Muñoz, A.D.; Ponce-Guarneros, M.; Gamez-Nava, J.I.; Olivas-Flores, E.M.; Mejia, M.; Juárez-Contreras, P.; Martínez-García, E.A.; Corona-Sanchez, E.G.; Rodríguez-Hernández, T.M.; Mercado, M.V.-D.; et al. Anti-Cyclic Citrullinated Peptide Antibodies and Severity of Interstitial Lung Disease in Women with Rheumatoid Arthritis. J. Immunol. Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Bombardieri, M.; Del Papa, N.; Cinquini, M.; Magrini, L.; Tincani, A.; Valesini, G. Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFα therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann. Rheum. Dis. 2004, 63, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.A.; Lin, K.C.; Chen, C.H.; Liao, H.T.; Wang, H.P.; Chang, H.N.; Tsai, C.Y.; Chou, C. The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005, 65, 35–39. [Google Scholar] [CrossRef]
- Lal, P.; Su, Z.; Holweg, C.T.J.; Silverman, G.J.; Schwartzman, S.; Kelman, A.; Read, S.; Spaniolo, G.; Monroe, J.G.; Behrens, T.W.; et al. Inflammation and autoantibody markers identify rheumatoid arthritis patients with enhanced clinical benefit following rituximab treatment. Arthritis Rheum. 2011, 63, 3681–3691. [Google Scholar] [CrossRef]
- Váncsa, A.; Szabó, Z.; Szamosi, S.; Bodnár, N.; Végh, E.; Gergely, L.; Szücs, G.; Szántó, S.; Szekanecz, Z. Longterm Effects of Rituximab on B Cell Counts and Autoantibody Production in Rheumatoid Arthritis: Use of High-sensitivity Flow Cytometry for More Sensitive Assessment of B Cell Depletion. J. Rheumatol. 2013, 40, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Smolen, J.S. The rheumatoid arthritis patient in the clinic: Comparing more than 1300 consecutive DMARD courses. Rheumatology 2002, 41, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Tishler, M.; Caspi, D.; Yaron, M. C-Reactive protein levels in patients with rheumatoid arthritis: The impact of therapy. Clin. Rheumatol. 1985, 4, 321–324. [Google Scholar] [CrossRef]
- Jeurissen, M.E.C.; Boerbooms, A.M.T.; Van De Putte, L.B.A.; Doesburg, W.H.; Mulder, J.; Rasker, J.J.; Kruijsen, M.W.M.; Haverman, J.F.; Van Beusekom, H.J.; Muller, W.H.; et al. Methotrexate versus azathioprine in the treatment of rheumatoid arthritis. A forty-eight–week randomized, double-blind trial. Arthritis Rheum. 1991, 34, 961–972. [Google Scholar] [CrossRef]
- Taaning, E.; Simonsen, A.C.; Hjelms, E.; Svejgaard, A.; Morling, N. Platelet Alloimmunization after Transfusion. A Prospective Study in 117 Heart Surgery Patients. Vox Sang. 1997, 72, 238–241. [Google Scholar] [CrossRef]
- Itescu, S.; Tung, T.C.; Burke, E.M.; Weinberg, A.; Moazami, N.; Artrip, J.H.; Suciu-Foca, N.; Rose, E.A.; Oz, M.; Michler, R.E. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation 1998, 98, 786–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugière, O.; Suberbielle, C.; Thabut, G.; Lhuillier, E.; Dauriat, G.; Métivier, A.-C.; Gautreau, C.; Charron, M.; Mal, H.; Parquin, F.; et al. Lung Transplantation in Patients with Pretransplantation Donor-Specific Antibodies Detected by Luminex Assay. Transplantation 2013, 95, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Wiwattanathum, P.; Ingsathit, A.; Thammanichanond, D.; Mongkolsuk, T.; Sumethkul, V. Significance of HLA Antibody Detected by PRA-Bead Method in Kidney Transplant Outcomes. Transplant. Proc. 2016, 48, 761–765. [Google Scholar] [CrossRef]
- Roubinian, N. TACO and TRALI: Biology, risk factors, and prevention strategies. Am. Soc. Hematol. 2018, 2018, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, H.; Oka, S.; Shimada, K.; Masuo, K.; Nakajima, F.; Funano, S.; Tanaka, Y.; Komiya, A.; Fukui, N.; Sawasaki, T.; et al. Autoantibody Profiles in Collagen Disease Patients with Interstitial Lung Disease (ILD): Antibodies to Major Histocompatibility Complex Class I-Related Chain A (MICA) as Markers of ILD. Biomark. Insights 2015, 10, 63–73. [Google Scholar] [CrossRef]
- Jackman, R.I.; Cruz, G.; Nititham, J.; Triulzi, D.J.; Barcellos, L.F.; Criswell, L.A.; Norris, P.J.; Busch, M.P. Increased alloreactive and autoreactive antihuman leucocyte antigen antibodies associated with systemic lupus erythematosus and rheumatoid arthritis. Lupus Sci. Med. 2018, 5, e000278. [Google Scholar] [CrossRef] [Green Version]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef]
- Bay-Jensen, A.-C.; Platt, A.; Jenkins, M.A.; Weinblatt, M.E.; Byrjalsen, I.; Musa, K.; Genovese, M.C.; Karsdal, M.A. Tissue metabolite of type I collagen, C1M, and CRP predicts structural progression of rheumatoid arthritis. BMC Rheumatol. 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Takao, S.; Masuda, T.; Yamaguchi, K.; Sakamoto, S.; Horimasu, Y.; Nakashima, T.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; et al. High preoperative C-reactive protein level is a risk factor for acute exacerbation of interstitial lung disease after non-pulmonary surgery. Medicine 2019, 98, e14296. [Google Scholar] [CrossRef]
- Elias, T.; Zahava, V. Innate immune-responses and their role in driving autoimmunity. Autoimmun. Rev. 2019, 18, 306–311. [Google Scholar] [CrossRef]
- Du Clos, T.W.; Mold, C. C-reactive Protein: An Activator of Innate Immunity and a Modulator of Adaptive Immunity. Immunol Res. 2004, 30, 261–277. [Google Scholar] [CrossRef]


| Variable | RA-ILD n = 65 | RA n = 82 | p-value |
|---|---|---|---|
| Female (%) | 53 (81.54) | 81 (98.78) | 0.002 |
| Age | 61 (37–85) | 53.50 (25–80) | <0.001 |
| Age at RA diagnosis | 53 (23–85) | 45.50 (18–75) | <0.001 |
| Age at ILD diagnosis | 59 (37–85) | NA | |
| FEV1* (%) | 66 (18–145) | 98 (48–134) | <0.001 |
| FVC* (%) | 67 (26–147) | 97 (54–128) | <0.001 |
| FEV1/FVC* (%) | 82 (30–110) | 99 (58–117) | <0.001 |
| anti-CCP+, n (%) | 49 (83.05) | 59 (95.16) | |
| anti-CCP+ (UI/mL) | 190.91 (44.77–378) | 322.6 (42.5–531) | <0.001 |
| RF+, n (%) | 56 (94.92) | 61 (93.85) | |
| RF+ (UI/mL) | 291 (20.20–4550) | 182 (20.40–2100) | |
| CRP (mg/dL) | 1.56 (0.01–32) | 1.40 (0.05–24) | |
| ESR (mm/h) | 32 (1–45) | 26 (2–110) | |
| PRA+, n (%) | 16 (24.62) | 24 (29.27) | |
| SDAI | 28.10 (1–76.01) | 32.25 (1.31–100.0) | |
| Exposure | |||
| Tobacco smoking, n (%) | 22 (37.93) | 19 (31.67) | 0.003 |
| Tobacco index | 5.28 (0.10–78) | 2 (0.20–10.50) | 0.04 |
| Biomass-burning exposition, n (%) | 23 (43.40) | 16 (30.77) | |
| Biomass-burning exposition index | 79 (14–638) | 34 (3–414) | 0.014 |
| Exp-Org, n (%) | 33 (52.38) | 36 (48.65) | |
| Exp-Inor, n (%) | 21 (33.87) | 24 (31.17) | |
| Treatment | |||
| Methotrexate, n (%) | 59 (93.65) | 76 (97.44) | |
| Leflunomide, n (%) | 27 (42.86) | 22 (28.21) | |
| Sulfasalazine, n (%) | 12 (19.05) | 51 (65.38) | <0.001 |
| Cloroquine/ Hydroxychloroquine, n (%) | 22 (34.92) | 54 (69.23) | <0.001 |
| bDMARDs, n (%) | 6 (9.52) | 1 (1.28) | |
| Azathioprine, n (%) | 8 (12.70) | 0 | |
| Prednisone, n (%) | 44 (69.84) | 31 (39.74) | 0.001 |
| Variable | RA-ILD n = 16 | RA n = 24 | p-Value |
|---|---|---|---|
| Female (%) | 15 (93.75) | 24 (100) | |
| Age at RA diagnosis | 52 (32–85) | 46.5 (23–69) | |
| FEV1* (%) | 61 (25–145) | 94 (62–129) | <0.001 |
| FVC* (%) | 62 (26–147) | 97 (77–126) | <0.001 |
| FEV1/FVC* (%) | 82.25 (49–93.30) | 94 (58–117) | <0.004 |
| anti-CCP+, n (%) | 16 (100.0) | 17 (89.47) | |
| anti-CCP+ (UI/mL) | 195.96 (56.5–345.6) | 244.25 (63.2–501) | 0.032 |
| FR+, n (%) | 16 (100) | 16 (94.12) | |
| FR+ (UI/mL) | 242 (34.9–2970) | 220.6 (20.4–1647.7) | |
| CRP (mg/dL) | 1.94 (0.01–32 | 1.38 (0.05–24) | |
| ESR (mm/h) | 35.5 (1.49–40) | 29 (2–110) | |
| Tobacco smoking, n (%) | 5 (33.33) | 3 (16.67) | |
| Tobacco index | 7.3 (0.10–23.5) | 2.4 (0.80–5.0) | |
| Biomass-burning exposition, n (%) | 8 (61.54) | 4 (23.53) | |
| Biomass-burning exposition index | 99.5 (25–400) | 32.5 (14–414) | |
| Exp-Org, n (%) | 7 (46.67) | 10 (41.67) | |
| Exp-Inor, n (%) | 4 (28.57) | 5 (21.74) | |
| PRA (%) | 20 (3–71) | 23 (3–89) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Angel-Pablo, A.D.; Buendía-Roldán, I.; Mejía, M.; Pérez-Rubio, G.; Nava-Quiroz, K.J.; Rojas-Serrano, J.; Falfán-Valencia, R. Anti-HLA Class II Antibodies Correlate with C-Reactive Protein Levels in Patients with Rheumatoid Arthritis Associated with Interstitial Lung Disease. Cells 2020, 9, 691. https://doi.org/10.3390/cells9030691
Del Angel-Pablo AD, Buendía-Roldán I, Mejía M, Pérez-Rubio G, Nava-Quiroz KJ, Rojas-Serrano J, Falfán-Valencia R. Anti-HLA Class II Antibodies Correlate with C-Reactive Protein Levels in Patients with Rheumatoid Arthritis Associated with Interstitial Lung Disease. Cells. 2020; 9(3):691. https://doi.org/10.3390/cells9030691
Chicago/Turabian StyleDel Angel-Pablo, Alma D., Ivette Buendía-Roldán, Mayra Mejía, Gloria Pérez-Rubio, Karol J. Nava-Quiroz, Jorge Rojas-Serrano, and Ramcés Falfán-Valencia. 2020. "Anti-HLA Class II Antibodies Correlate with C-Reactive Protein Levels in Patients with Rheumatoid Arthritis Associated with Interstitial Lung Disease" Cells 9, no. 3: 691. https://doi.org/10.3390/cells9030691