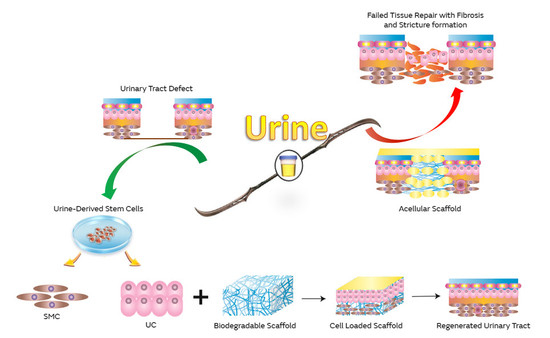
Urine as a Main Effector in Urological Tissue Engineering—A Double-Edged Sword
Abstract
:1. Clinical Need
2. Urothelium as a Urine Barrier
3. Bioengineered Urothelium
4. Urine-Derived Stem Cells (UDSCs)
4.1. Origin of Urine-Derived Stem Cells
4.2. Advantages of UDSCs
4.2.1. Cell Proliferation and Differentiation
4.2.2. Self-Renewal Capability
4.2.3. Colony Formation
4.2.4. Vascularization Ability of UDSCs
4.2.5. Extracellular Vesicles Secreted by UDSCs
4.3. Markers of UDSCs
4.4. Limitations of UDSCs
5. Urine-Derived Stem Cells in Urethral Regeneration
6. Urine Cytotoxicity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aitken, K.J.; Bägli, D.J. The bladder extracellular matrix. Part I: Architecture, development and disease. Nat. Rev. Urol. 2009, 6, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.E. Urinary Diversion: Ileal Conduit to Neobladder. J. Urol. 2003, 169, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Sedberry-Ross, S.; Stisser, B.C.; Henderson, C.G.; Rushton, H.G.; Belman, A.B. Split Prepuce In Situ Onlay Hypospadias Repair: 17 Years of Experience. J. Urol. 2007, 178, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Barbagli, G.; Kulkarni, S.B.; Fossati, N.; Larcher, A.; Sansalone, S.; Guazzoni, G.; Romano, G.; Pankaj, J.; Dell’Acqua, V.; Lazzeri, M. Long-Term Followup and Deterioration Rate of Anterior Substitution Urethroplasty. J. Urol. 2014, 192, 808–813. [Google Scholar] [CrossRef]
- Xu, Y.M.; Li, C.; Xie, H.; Sa, Y.L.; Fu, Q.; Wu, D.L.; Zhang, J.; Feng, C.; Jin, C.R. Intermediate-Term Outcomes and Complications of Long Segment Urethroplasty with Lingual Mucosa Grafts. Intermediate-Term Outcomes and Complications of Long Segment Urethroplasty with Lingual Mucosa Grafts. J. Urol. 2017, 198, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.O.; Mahdi, E.; Hasan, A.; AlAnsari, A.; Pennisi, C.P. Current Status of Tissue Engineering in the Management of Severe Hypospadias. Front. Pediatr. 2018, 5, 283. [Google Scholar] [CrossRef] [Green Version]
- Olesen, K.P.; Walter, S.; Frimodt-Moller, C.; Hebjorn, S.; Gammelgaard, P.A.; Hald, T. Morphology and function of the bladder and urethra in female urinary incontinence. Int. Urol. Nephrol. 1975, 7, 303–313. [Google Scholar] [CrossRef]
- Mawhinney, M.; Mariotti, A. Physiology, pathology and pharmacology of the male reproductive system. Periodontology 2013, 61, 232–251. [Google Scholar] [CrossRef]
- Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 1763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Frimberger, D.; Cheng, E.Y.; Lin, H.; Kropp, B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. Bju Int. 2006, 98, 1100–1105. [Google Scholar] [CrossRef]
- De Filippo, R.E.; Yoo, J.J.; Atala, A. Urethral replacement using cell seeded tubularized collagen matrices. J. Urol. 2002, 168, 1789–1793. [Google Scholar] [CrossRef]
- Aitken, K.J.; Bägli, D.J. The bladder extracellular matrix. Part II: Regenerative applications. Nat. Rev. Urol. 2009, 6, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bivalacqua, T.J.; Sopko, N. Urinary Tissue Engineering: Challenges and Opportunities. Sex Med. Rev. 2018, 6, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Eberli, D.; Gobet, R.; Salemi, S. Tissue Engineering in Pediatric Bladder Reconstruction-The Road to Success. Front. Pediatr. 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birder, L.; Andersson, K.-E. Urothelial Signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkad, T.; Pelzer, A.E.; Mitterberger, M.; Rehder, P.; Leonhartsberger, N.; Bartsch, G.; Strasser, H. Influence of intravesical potassium on pelvic floor activity in women with recurrent urinary tract infections: Comparative urodynamics might lead to enhanced detection of dysfunctional voiding. BJU Int. 2007, 100, 1071–1074. [Google Scholar] [CrossRef]
- Pokrywczyńska, M.; Kloskowski, T.; Balcerczyk, D.; Buhl, M.; Jundziłł, A.; Nowacki, M.; Męcińska-Jundziłł, K.; Drewa, T. Stem cells and differentiated cells differ in their sensitivity to urine in vitro. J. Cell Biochem. 2018, 119, 2307–2319. [Google Scholar] [CrossRef]
- Rajasekaran, M.; Stein, P.; Parsons, C.L. Toxic factors in human urine that injure urothelium. Int. J. Urol. 2006, 13, 409–414. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Sabbagh, W.; Masters, J.R.; Duffy, P.G.; Herbage, D.; Brown, R.A. In vitro assessment of a collagen sponge for engineering urothelial grafts. Br. J. Urol. 1998, 82, 888–894. [Google Scholar] [CrossRef]
- Gandhi, D.; Molotkov, A.; Batourina, E.; Schneider, K.; Dan, H.; Reiley, M.; Laufer, E.; Metzger, D.; Liang, F.; Liao, Y.; et al. Retinoid Signaling in Progenitors Controls Specification and Regeneration of the Urothelium. Dev. Cell 2013, 26, 469–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, K.; Lee, J.; Guo, N.; Kim, J.; Lim, A.; Qu, L.; Mysorekar, I.U.; Beachy, P.A. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 2011, 472, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, J.; Li, C.; Li, H.; Chang, J. Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology 2011, 63, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yu, H.; Fan, C.; Kong, Q.; Liu, D.; Meng, L. Differentiate into urothelium and smooth muscle cells from adipose tissue-derived stem cells for ureter reconstruction in a rabbit model. Am. J. Transl. Res. 2016, 8, 3757–3768. [Google Scholar]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine Derived Cells are a Potential Source for Urological Tissue Reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef]
- Oottamasathien, S.; Wang, Y.; Williams, K.; Franco, O.E.; Wills, M.L.; Thomas, J.C.; Saba, K.; Sharif-Afshar, A.R.; Makari, J.H.; Bhowmick, N.A. Directed differentiation of embryonic stem cells into bladder tissue. Dev. Biol. 2007, 304, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Kim, H.; Han, Y.-M. Generation of Bladder Urothelium from Human Pluripotent Stem Cells under Chemically Defined Serum- and Feeder-Free System. Int. J. Mol. Sci. 2014, 15, 7139–7157. [Google Scholar] [CrossRef] [Green Version]
- Wan, Q.; Xiong, G.; Liu, G.; Shupe, T.D.; Wei, G.; Zhang, D.; Lu, X.; Atala, A.; Zhang, Y. Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction. Stem Cell Res. 2018, 9, 304. [Google Scholar] [CrossRef]
- Anumanthan, G.; Makari, J.H.; Honea, L.; Thomas, J.C.; Wills, M.L.; Bhowmick, N.A.; Adams, M.C.; Hayward, S.W.; Matusik, R.J.; Brock, J.W., 3rd; et al. Directed Differentiation of Bone Marrow Derived Mesenchymal Stem Cells Into Bladder Urothelium. J. Urol. 2008, 180, 1778–1783. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Long, T.; Deng, J.; Zhang, Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res. 2014, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014, 1, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayhan, S.E.; Tezcan Keles, G.; Topcu, I.; Mir, E.; Ismet Deliloglu Gurhan, S. Isolation and in vitro cultivation of human urine-derived cells: An alternative stem cell source. Turk. J. Urol. 2017, 43, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J. Cell Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.-E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Kim, H.T.; Lee, J.-S.; Kim, M.J.; Kim, B.S.; Kim, B.W.; Kwon, T.G. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology 2012, 79, 1186.e1-7. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Choi, S.H.; Kim, B.S.; Choi, J.Y.; Park, G.-B.; Kwon, T.G.; Chun, S.Y. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J. Korean Med. Sci. 2015, 30, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Pavathuparambil Abdul Manaph, N.; Al-Hawwas, M.; Bobrovskaya, L.; Coates, P.T.; Zhou, X.-F. Urine-derived cells for human cell therapy. Stem Cell Res. 2018, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856. [Google Scholar] [CrossRef]
- Lang, R.; Liu, G.; Shi, Y.; Bharadwaj, S.; Leng, X.; Zhou, X.; Liu, H.; Atala, A.; Zhang, Y. Self-Renewal and Differentiation Capacity of Urine-Derived Stem Cells after Urine Preservation for 24 Hours. PLoS ONE 2013, 8, e53980. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Z.; Bharadwaj, S.; Hodges, S.J.; Atala, A.; Zhang, Y. Implantation of Autologous Urine Derived Stem Cells Expressing Vascular Endothelial Growth Factor for Potential Use in Genitourinary Reconstruction. J. Urol. 2011, 186, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Pareta, R.A.; Wu, R.; Shi, Y.; Zhou, X.; Liu, H.; Deng, C.; Sun, X.; Atala, A.; Opara, E.C.; et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials 2013, 34, 1311–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Cai, X.; Wang, L.; Liao, B.; Zhang, H.; Shan, Y.; Chen, Q.; Zhou, T.; Li, X.; Hou, J.; et al. Generating a Non-Integrating Human Induced Pluripotent Stem Cell Bank from Urine-Derived Cells. PLoS ONE 2013, 8, e70573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Citra, F.; Wang, D.-A. Prospects of induced pluripotent stem cell technology in regenerative medicine. Tissue Eng. Part B 2011, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kropp, B.P.; Moore, P.; Cowan, R.; Furness, P.D.; Kolligian, M.E.; Frey, P.; Cheng, E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J. Urol. 2000, 164, 928–935. [Google Scholar] [CrossRef]
- Zhang, Y.; Kropp, B.P.; Lin, H.-K.; Cowan, R.; Cheng, E.Y. Bladder Regeneration with Cell-Seeded Small Intestinal Submucosa. Tissue Eng. 2004, 10, 181–187. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, X.; Shindel, A.W.; Ning, H.; Ferretti, L.; Jin, X.; Lin, G.; Lin, C.S.; Lue, T.F. Adipose Tissue-Derived Stem Cells Ameliorate Diabetic Bladder Dysfunction in a Type II Diabetic Rat Model. Stem Cells Dev. 2012, 21, 1391–1400. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.-R.; Song, Y.S.; Lee, H.J. Stem Cell Therapy in Bladder Dysfunction: Where Are We? And Where Do We Have to Go? Biomed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gnecchi, M.; Melo, L.G. Bone Marrow-Derived Mesenchymal Stem Cells: Isolation, Expansion, Characterization, Viral Transduction, and Production of Conditioned Medium. In: Methods in molecular biology. Stem Cells Regener. Med. 2009, 482, 281–294. [Google Scholar]
- Ayatollahi, M.; Geramizadeh, B.; Zakerinia, M.; Ramzi, M.; Yaghobi, R.; Hadadi, P.; Rezvani, A.R.; Aghdai, M.; Azarpira, N.; Karimi, H. Human Bone Marrow-derived Mesenchymal Stem Cell: A Source for Cell-Based Therapy. Int. J. Organ Transpl. Med. 2012, 3, 32–41. [Google Scholar]
- Tian, H.; Bharadwaj, S.; Liu, Y.; Ma, H.; Ma, P.X.; Atala, A.; Zhang, Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials 2010, 31, 870–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloskowski, T.; Nowacki, M.; Pokrywczyńska, M.; Drewa, T. Urine—A waste or the future of regenerative medicine? Med. Hypotheses 2015, 84, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, J.; Meyers, S.; Ramage, R.; Bastacky, S.; Doty, D.; Apodaca, G.; Zeidel, M.L. Bladder permeability barrier: Recovery from selective injury of surface epithelial cells. Am. J. Physiol. Physiol. 2002, 283, F242–F253. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Wang, M.; Chen, F.; Zhou, J. Urine-Derived Stem Cells: The Present and the Future. Stem Cells Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Han, X.; Chen, Z.; Fang, J.; Huang, X.; Wei, H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res. 2018, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lei, H.; Xu, Y.; Li, H.; Yang, B.; Yu, C.; Yuan, Y.; Fang, D.; Xin, Z.; Guan, R. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology 2018, 6, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, B.; Xie, Y.; Zhang, C.; Deng, C.; Lv, L.; Yao, J.; Zhang, Y.; Liu, G.; Deng, J.; Deng, C. Extracellular Vesicles From Human Urine-Derived Stem Cells Ameliorate Erectile Dysfunction in a Diabetic Rat Model by Delivering Proangiogenic MicroRNA. Sex Med. 2019, 7, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Prattichizzo, F.; Giuliani, A.; De Nigris, V.; Pujadas, G.; Ceka, A.; La Sala, L.; Genovese, S.; Testa, R.; Procopio, A.D.; Olivieri, F. Extracellular microRNAs and endothelial hyperglycaemic memory: A therapeutic opportunity? Diabetes Obes. Metab. 2016, 18, 855–867. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Q.; Niu, X.; Zhang, G.; Ling, X.; Zhang, J.; Wang, Y.; Deng, Z. Extracellular Vesicles Secreted by Human Urine-Derived Stem Cells Promote Ischemia Repair in a Mouse Model of Hind-Limb Ischemia. Cell Physiol. Biochem. 2018, 47, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S. Advances in stem cell therapy for the lower urinary tract. World J. Stem Cells 2010, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-Seeded Tubularized Scaffolds for Reconstruction of Long Urethral Defects: A Preclinical Study. Eur. Urol. 2013, 63, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Žiaran, S.; Galambošová, M.; Danišovič, L. Tissue engineering of urethra: Systematic review of recent literature. Exp. Biol. Med. 2017, 242, 1772–1785. [Google Scholar] [CrossRef]
- Culenova, M.; Ziaran, S.; Danisovic, L. Cells Involved in Urethral Tissue Engineering: Systematic Review. Cell Transplant. 2019. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, B.; Deng, J.; Zhuang, G.; Wu, S.; Liu, G.; Deng, C.; Yang, G.; Qiu, X.; Wei, P. Characterization of rabbit urine-derived stem cells for potential application in lower urinary tract tissue regeneration. Cell Tissue Res. 2018, 374, 303–315. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res. 2017, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bharadwaj, S.; Lee, S.J.; Atala, A.; Zhang, Y. Optimization of a natural collagen scaffold to aid cell–matrix penetration for urologic tissue engineering. Biomaterials 2009, 30, 3865–3873. [Google Scholar] [CrossRef]
- Singh, M.; Blandy, J.P. The pathology of urethral stricture. J. Urol. 1976, 115, 673–676. [Google Scholar] [CrossRef]
- Davis, N.F.; Callanan, A.; McGuire, B.B.; Flood, H.D.; McGloughlin, T.M. Evaluation of Viability and Proliferative Activity of Human Urothelial Cells Cultured Onto Xenogenic Tissue-Engineered Extracellular Matrices. Urology 2011, 77, 1007.e1–1007.e7. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Kloskowski, T.; Tworkiewicz, J.; Pokrywczyńska, M.; Drewa, T. Urine Is a Highly Cytotoxic Agent: Does It Influence Stem Cell Therapies in Urology? Transpl. Proc. 2012, 44, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Trécherel, E.; Godin, C.; Louandre, C.; Benchitrit, J.; Poirot, S.; Mazière, J.C.; Massy, Z.A.; Galmiche, A. Upregulation of BAD, a pro-apoptotic protein of the BCL2 family, in vascular smooth muscle cells exposed to uremic conditions. Biochem. Biophys. Res. Commun. 2012, 417, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Glinos, A.D.; Bardi, G.N.; Dermitzaki, K.C.; Perez, S.A.; Talieri, M.J. Cytokinetic and cytotoxic effects of urea on HeLa cells in suspension cultures. J. Natl. Cancer Inst. 1983, 71, 1211–1219. [Google Scholar] [PubMed]



| Features | Cell Type | ||||
|---|---|---|---|---|---|
| UDSCs [25,35,38,39,40,41,42] | iPSCs/ESC [43,44] | Bladder SMC, UCs [45,46] | ADSCs [47,48] | BMSCs [23,29,49,50,51] | |
| Harvesting technique | Non-invasive, Low-cost, Easy | Invasive | Invasive | Invasive | Invasive |
| Stem cell isolation | Very Easy | Easy | N/A | Neutral | Neutral |
| Angiogenic trophic factors | Yes | Unknown | Limited | Yes | Yes |
| Immuno-modulatory properties | Yes | Unknown | Unknown | Yes | Yes |
| Oncogenic potential | No | Yes | No | No | No |
| Telomerase activity | 75% of UDSC have Telomerase Activity | Have Telomerase Activity | No Telomerase Activity | Unknown | Unknown |
| Endothelial and urothelial differentiation capability | High Ability (60–85%) | Yes | N/A | Yes | Yes |
| Multi-lineage differentiation capability | Multipotent | Pluri-potent | N/A | Multipotent (mesodermal cell lineages) | Multipotent (mesodermal cell lineages) |
| Self-renewal capability | High Capability | Strong Capability | Capable | Unknown | Capable |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, T.O.; Ali, T.A.; Uddin, S. Urine as a Main Effector in Urological Tissue Engineering—A Double-Edged Sword. Cells 2020, 9, 538. https://doi.org/10.3390/cells9030538
Abbas TO, Ali TA, Uddin S. Urine as a Main Effector in Urological Tissue Engineering—A Double-Edged Sword. Cells. 2020; 9(3):538. https://doi.org/10.3390/cells9030538
Chicago/Turabian StyleAbbas, Tariq O., Tayyiba A. Ali, and Shahab Uddin. 2020. "Urine as a Main Effector in Urological Tissue Engineering—A Double-Edged Sword" Cells 9, no. 3: 538. https://doi.org/10.3390/cells9030538