Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Maternal Administration
2.3. Immediate Neonatal Administration
2.4. Measurement of SR59230A Blood Concentration
2.5. Real Time RT-PCR
2.6. Flow Cytometry Analysis
2.7. Histological Staining and Morphometrical Analysis
2.8. Determination of Vessel Caliber
2.9. Statistical Analysis
3. Results
3.1. SR59230A Blood Concentration
3.2. β3-ARs Expression on DA
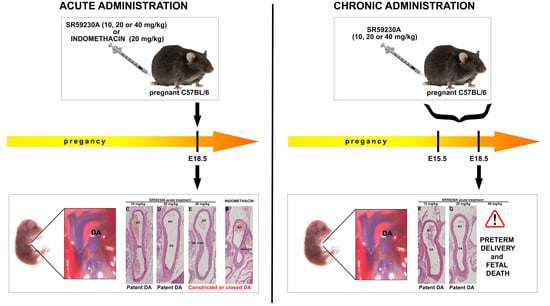
3.3. Response of the Fetal DA to Acute SR59230A Exposure
3.4. Response of the Fetal DA to Chronic SR59230A Exposure
3.5. Newborn DA Closure after SR59230A Exposure In Utero
3.6. Response of the Neonatal DA to BRL37344 Exposure
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular characterization of the human beta 3-adrenergic receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Nantel, F.; Bonin, H.; Emorine, L.J.; Zilberfarb, V.; Strosberg, A.D.; Bouvier, M.; Marullo, S. The human beta 3-adrenergic receptor is resistant to short term agonist-promoted desensitization. Mol. Pharmacol. 1993, 43, 548–555. [Google Scholar]
- Dal Monte, M.; Filippi, L.; Bagnoli, P. Beta3-adrenergic receptors modulate vascular endothelial growth factor release in response to hypoxia through the nitric oxide pathway in mouse retinal explants. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Loustalot, C.; Cortijo, J.; Simon, B.; Naline, E.; Dumas, M.; Esteve, S.; Croci, T.; Chalon, P.; Frydman, R.; et al. Functional, biochemical and molecular biological evidence for a possible beta(3)-adrenoceptor in human near-term myometrium. Br. J. Pharmacol. 2000, 130, 1960–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyer, C.; Gautier, J.F.; Danforth, E.J. Development of beta 3-adrenoceptor agonists for the treatment of obesity and diabetes—an update. Diabetes Metab. 1999, 25, 11–21. [Google Scholar] [PubMed]
- Grujic, D.; Susulic, V.S.; Harper, M.E.; Himms-Hagen, J.; Cunningham, B.A.; Corkey, B.E.; Lowell, B.B. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J. Biol. Chem. 1997, 272, 17686–17693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursino, M.G.; Vasina, V.; Raschi, E.; Crema, F.; De Ponti, F. The beta3-adrenoceptor as a therapeutic target: Current perspectives. Pharmacol. Res. 2009, 59, 221–234. [Google Scholar] [CrossRef]
- Coman, O.A.; Păunescu, H.; Ghiţă, I.; Coman, L.; Bădărăru, A.; Fulga, I. Beta 3 adrenergic receptors: Molecular, histological, functional and pharmacological approaches. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2009, 50, 169–179. [Google Scholar]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about β(3)-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, C.; Rozec, B.; Manoury, B.; Balligand, J.-L. Beta-3 adrenoceptors as new therapeutic targets for cardiovascular pathologies. Curr. Heart Fail. Rep. 2011, 8, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Oriowo, M.A. Atypical beta-adrenoceptors in the rat isolated common carotid artery. Br. J. Pharmacol. 1994, 113, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Oriowo, M.A. Different atypical beta-adrenoceptors mediate isoprenaline-induced relaxation in vascular and non-vascular smooth muscles. Life Sci. 1995, 56, PL269–PL275. [Google Scholar] [CrossRef]
- Mori, A.; Miwa, T.; Sakamoto, K.; Nakahara, T.; Ishii, K. Pharmacological evidence for the presence of functional beta(3)-adrenoceptors in rat retinal blood vessels. Naunyn. Schmiedebergs Arch. Pharmacol. 2010, 382, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.J.; McGrath, J.C. Previously unsuspected widespread cellular and tissue distribution of beta-adrenoceptors and its relevance to drug action. Trends Pharmacol. Sci. 2011, 32, 219–226. [Google Scholar] [CrossRef]
- Dal Monte, M.; Casini, G.; Filippi, L.; Nicchia, G.P.; Svelto, M.; Bagnoli, P. Functional involvement of beta3-adrenergic receptors in melanoma growth and vascularization. J. Mol. Med. Berl. 2013, 91, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Bruno, G.; Dal Monte, M. beta3 -Adrenoceptor as a potential immuno-suppressor agent in melanoma. Int. J. Mol. Sci. 2019, 176, 2149. [Google Scholar] [CrossRef] [Green Version]
- Calvani, M. beta3-Adrenoreceptors Control Mitochondrial Dormancy in Melanoma and Embryonic Stem Cells. Br. J. Pharmacol. 2018, 2018, 6816508. [Google Scholar] [CrossRef]
- Bruno, G.; Cencetti, F.; Pini, A.; Tondo, A.; Cuzzubbo, D.; Fontani, F.; Strinna, V.; Buccoliero, A.M.; Casazza, G.; Donati, C.; et al. β3-adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P(2) modulation. Oncogene 2020, 39, 368–384. [Google Scholar] [CrossRef] [Green Version]
- Lamkin, D.M.; Sloan, E.K.; Patel, A.J.; Chiang, B.S.; Pimentel, M.A.; Ma, J.C.Y.; Arevalo, J.M.; Morizono, K.; Cole, S.W. Chronic stress enhances progression of acute lymphoblastic leukemia via beta-adrenergic signaling. Brain. Behav. Immun. 2012, 26, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Perrone, M.G.; Notarnicola, M.; Caruso, M.G.; Tutino, V.; Scilimati, A. Upregulation of beta3-adrenergic receptor mRNA in human colon cancer: A preliminary study. Oncology 2008, 75, 224–229. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlidis, N. Cancer and pregnancy: What should we know about the management with systemic treatment of pregnant women with cancer? Eur. J. Cancer Oxf. Engl. 1990 2011, 47 (Suppl. 3), S348–S352. [Google Scholar] [CrossRef]
- Rogers, J.E.; Dasari, A.; Eng, C. The Treatment of Colorectal Cancer During Pregnancy: Cytotoxic Chemotherapy and Targeted Therapy Challenges. Oncologist 2016, 21, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennedy, M.C.; Houlihan, D.D.; McMillan, H.; Morrison, J.J. Beta2- and beta3-adrenoreceptor agonists: Human myometrial selectivity and effects on umbilical artery tone. Am. J. Obstet. Gynecol. 2002, 187, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.A.; Hanson, K.; Mlynarczyk, M.; Kaushal, K.M.; Ducsay, C.A. Long-term hypoxia modulates expression of key genes regulating adipose function in the late-gestation ovine fetus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1312–R1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovalcik, V. The response of the isolated ductus arteriosus to oxygen and anoxia. J. Physiol. 1963, 169, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Heymann, M.A.; Rudolph, A.M. Control of the ductus arteriosus. Physiol. Rev. 1975, 55, 62–78. [Google Scholar] [CrossRef]
- Coceani, F.; Olley, P.M. The response of the ductus arteriosus to prostaglandins. Can. J. Physiol. Pharmacol. 1973, 51, 220–225. [Google Scholar] [CrossRef]
- Gournay, V. The ductus arteriosus: Physiology, regulation, and functional and congenital anomalies. Arch. Cardiovasc. Dis. 2011, 104, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.Y.; Brown, D.M. Effect of dexamethasone on fetal lung 15-hydroxy-prostaglandin dehydrogenase: Possible mechanism for the prevention of patent ductus arteriosus by maternal dexamethasone therapy. Prostaglandins. Leukot. Med. 1987, 27, 237–245. [Google Scholar] [CrossRef]
- Padrini, L.; Isacchi, B.; Bilia, A.R.; Pini, A.; Lanzi, C.; Masini, E.; Della Bona, M.L.; Calvani, A.M.; Ceccantini, R.; la Marca, G.; et al. Pharmacokinetics and local safety profile of propranolol eye drops in rabbits. Pediatr. Res. 2014, 76, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, J.; Anderson, J.D.; Brown, N.; Roman, C.; Clyman, R.I. Inhibition of cyclooxygenase isoforms in late- but not midgestation decreases contractility of the ductus arteriosus and prevents postnatal closure in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1717–R1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takami, T.; Momma, K.; Imamura, S. Increased constriction of the ductus arteriosus by dexamethasone, indomethacin, and rofecoxib in fetal rats. Circ. J. Off. J. Jpn. Circ. Soc. 2005, 69, 354–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujinaga, M.; Scott, J.C. Gene expression of catecholamine synthesizing enzymes and beta adrenoceptor subtypes during rat embryogenesis. Neurosci. Lett. 1997, 231, 108–112. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G. Beta3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. Int. J. Mol. Sci. 2020, 21, 1420. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Roman, C.; Chemtob, S.; Tse, M.M.; Lin, E.; Heymann, M.A.; Clyman, R.I. Cyclooxygenase-2 inhibitors constrict the fetal lamb ductus arteriosus both in vitro and in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1496–R1505. [Google Scholar] [CrossRef]
- Loftin, C.D.; Trivedi, D.B.; Langenbach, R. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J. Clin. Investg. 2002, 110, 549–557. [Google Scholar] [CrossRef]
- Yokoyama, U.; Minamisawa, S.; Shioda, A.; Ishiwata, R.; Jin, M.-H.; Masuda, M.; Asou, T.; Sugimoto, Y.; Aoki, H.; Nakamura, T.; et al. Prostaglandin E2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation 2014, 129, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Coceani, F.; Baragatti, B. Mechanisms for ductus arteriosus closure. Semin. Perinatol. 2012, 36, 92–97. [Google Scholar] [CrossRef]
- Patra, S.; Biswas, B.; Patra, R. Constriction of the Umbilical Cord by an Amniotic Band Leading to Fetal Demise. J. Obstet. Gynaecol. India 2015, 65, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Rouget, C.; Bardou, M.; Breuiller-Fouché, M.; Loustalot, C.; Qi, H.; Naline, E.; Croci, T.; Cabrol, D.; Advenier, C.; Leroy, M.J. Beta3-adrenoceptor is the predominant beta-adrenoceptor subtype in human myometrium and its expression is up-regulated in pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 1644–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bexis, S.; Docherty, R.J. Role of alpha 1-and beta 3-adrenoceptors in the modulation by SR59230A of the effects of MDMA on body temperature in the mouse. Br. J. Pharmacol. 2009, 158, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Riemer, R.K.; Goldfien, A.; Roberts, J.M. Rabbit myometrial adrenergic sensitivity is increased by estrogen bur is independent of changes in alpha adrenoceptor concentration. J. Pharmacol. Exp. Ther. 1986, 240, 44–50. [Google Scholar]
- Calvani, M.; Dabraio, A.; Subbiani, A.; Buonvicino, D.; De Gregorio, V.; Ciullini Mannurita, S.; Pini, A.; Nardini, P.; Favre, C.; Filippi, L. β3-Adrenoceptors as Putative Regulator of Immune Tolerance in Cancer and Pregnancy. Front. Immunol. 2020, 11, 2098. [Google Scholar] [CrossRef] [PubMed]








| Primary Antibodies | Dilution | Producer |
|---|---|---|
| β3-AR | 1:20/1:100 | ab94506; Abcam, Cambridge, UK |
| CD31 | 1:20/1:100 | ab9498; Abcam, Cambridge, UK |
| α-SMA | 1:500 | ab21027; Abcam, Cambridge, UK |
| α-SMA | 1:50 | ab7817; Abcam, Cambridge, UK |
| Secondary Antibodies | Dilution | Producer |
| Alexa Fluor Anti-rabbit | 1:333 | Jackson Immuno Reasearch Labs, West Grove, PA, USA |
| Alexa Fluor Anti-mouse | 1:333 | Jackson Immuno Reasearch Labs, West Grove, PA, USA |
| Alexa Fluor Anti-goat | 1:333 | Jackson Immuno Reasearch Labs, West Grove, PA, USA |
| Treatment | Dam (μg/L) | Fetus (μg/L) |
|---|---|---|
| Acute 40 mg/kg | 13.1 ± 2.66 | 9.03 ± 2.01 |
| Acute 20 mg/kg | 4.88 ± 0.71 | 3.49 ± 0.53 |
| Acute 10 mg/kg | 2.5 ± 0.49 | 1.62 ± 0.29 |
| Chronic 20 mg/kg | 6.59 ± 0.88 | 4.47 ± 0.5 |
| Chronic 10 mg/kg | 3.51 ± 0.59 | 2.45 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pini, A.; Fazi, C.; Nardini, P.; Calvani, M.; Fabbri, S.; Guerrini, A.; Forni, G.; La Marca, G.; Rosa, A.C.; Filippi, L. Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus. Cells 2020, 9, 2625. https://doi.org/10.3390/cells9122625
Pini A, Fazi C, Nardini P, Calvani M, Fabbri S, Guerrini A, Forni G, La Marca G, Rosa AC, Filippi L. Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus. Cells. 2020; 9(12):2625. https://doi.org/10.3390/cells9122625
Chicago/Turabian StylePini, Alessandro, Camilla Fazi, Patrizia Nardini, Maura Calvani, Sergio Fabbri, Alessandro Guerrini, Giulia Forni, Giancarlo La Marca, Arianna Carolina Rosa, and Luca Filippi. 2020. "Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus" Cells 9, no. 12: 2625. https://doi.org/10.3390/cells9122625