MicroRNAs as Potential Pharmaco-Targets in Ischemia-Reperfusion Injury Compounded by Diabetes
Abstract
:1. Introduction
2. MicroRNAs (miRNAs)
2.1. MiR-34a
2.2. MiR-144
2.3. MiR-210
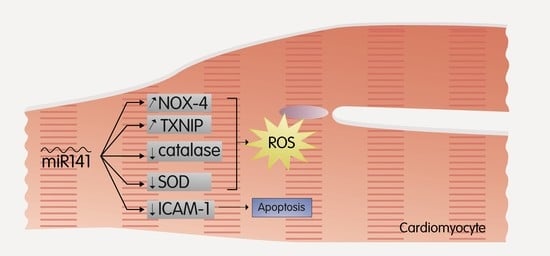
2.4. MiR-141
2.5. MiR-155
2.6. MiR-21 and MiR-146a
2.7. MiR-200c
2.8. Other Promising miRNAs as Therapeutic Targets
3. Conclusions
Funding
Conflicts of Interest
References
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, P.; Chiribiri, A.; Mancardi, D.; Rastaldo, R.; Gattullo, D.; Losano, G. Coronary endothelial dysfunction after ischemia and reperfusion and its prevention by ischemic preconditioning. Ital. Heart J. 2003, 4, 383–394. [Google Scholar] [PubMed]
- Folino, A.; Losano, G.; Rastaldo, R. Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. J. Cardiovasc. Pharmacol. 2013, 62, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maroszynska, I.; Fiedor, P. Leukocytes and endothelium interaction as rate limiting step in the inflammatory response and a key factor in the ischemia-reperfusion injury. Ann. Transplant. 2000, 5, 5–11. [Google Scholar] [PubMed]
- Haffner, S.M.; Lehto, S.; Ronnemaa, T.; Pyorala, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Liao, C.C.; Shih, C.C.; Yeh, C.C.; Chang, Y.C.; Hu, C.J.; Lin, J.G.; Chen, T.L. Impact of Diabetes on Stroke Risk and Outcomes: Two Nationwide Retrospective Cohort Studies. Medicine (Baltimore) 2015, 94, e2282. [Google Scholar] [CrossRef]
- WHO. The top 10 causes of death. World Health Organization. 2016. Available online: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 17 July 2018).
- IDF Diabetes Atlas Eighth Edition 2017. 2017. Available online: http://www.diabetesatlas.org/ (accessed on 12 November 2018).
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Radmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Hutvagner, G.; Mclachlan, J.; Pasquinelli, A.E.; Balint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell. Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Sermersheim, M.A.; Park, K.H.; Gumpper, K.; Adesanya, T.M.; Song, K.; Tan, T.; Ren, X.; Yang, J.M.; Zhu, H. MicroRNA regulation of autophagy in cardiovascular disease. Front. Biosci. (Landmark Ed.) 2017, 22, 48–65. [Google Scholar] [PubMed]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Curcio, A.; Indolfi, C. Emerging role of microRNAs in cardiovascular diseases. Circ. J. 2014, 78, 567–575. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Micheloni, S.; De Nigris, V.; Prattichizzo, F.; Ceriello, A. Novel insights into the regulation of miRNA transcriptional control: Implications for T2D and related complications. Acta Diabetol. 2018, 55, 989–998. [Google Scholar] [CrossRef]
- Kim, S.M.; Hur, D.Y.; Hong, S.W.; Kim, J.H. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem. Biophys. Res. Commun. 2017, 494, 550–555. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. The mechanism of long non-coding RNA MEG3 for hepatic ischemia-reperfusion: Mediated by miR-34a/Nrf2 signaling pathway. J. Cell Biochem. 2018, 119, 1163–1172. [Google Scholar] [CrossRef]
- Verjans, R.; Van Bilsen, M.; Schroen, B. MiRNA Deregulation in Cardiac Aging and Associated Disorders. Int. Rev. Cell Mol. Biol. 2017, 334, 207–263. [Google Scholar]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. MiR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef] [PubMed]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, L.; Li, F.; Hu, C.P.; Zhang, Z. Restoration of sirt1 function by pterostilbene attenuates hypoxia-reoxygenation injury in cardiomyocytes. Eur. J. Pharmacol. 2016, 776, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Lang, J.L.; Zhang, D.Y.; Sun, L.; Chen, W.; Liu, W.; Liu, K.Y.; Ma, C.Y.; Jiang, S.L.; Li, R.K.; et al. Suppression of miR-34a Expression in the Myocardium Protects Against Ischemia-Reperfusion Injury through SIRT1 Protective Pathway. Stem Cells Dev. 2017, 26, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Feng, Y.; Liu, D.; Zhang, Z.; Gao, K.; Zhang, W.; Tang, H. Thymoquinone Attenuates Myocardial Ischemia/Reperfusion Injury Through Activation of SIRT1 Signaling. Cell Physiol. Biochem. 2018, 47, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Quan, N.; Sun, W.; Chen, X.; Cates, C.; Rousselle, T.; Zhou, X.; Zhao, X.; Li, J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018, 114, 805–821. [Google Scholar] [CrossRef]
- Wang, G.; Yao, J.; Li, Z.; Zu, G.; Feng, D.; Shan, W.; Li, Y.; Hu, Y.; Zhao, Y.; Tian, X. MiR-34a-5p Inhibition Alleviates Intestinal Ischemia/Reperfusion-Induced Reactive Oxygen Species Accumulation and Apoptosis via Activation of SIRT1 Signaling. Antioxid. Redox Signal. 2016, 24, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Galimov, E.R. The Role of p66shc in Oxidative Stress and Apoptosis. Acta Nat. 2010, 2, 44–51. [Google Scholar]
- Wang, G.; Wang, J.J.; To, T.S.; Zhao, H.F.; Wang, J. Role of SIRT1-mediated mitochondrial and Akt pathways in glioblastoma cell death induced by Cotinus coggygria flavonoid nanoliposomes. Int. J. Nanomed. 2015, 10, 5005–5023. [Google Scholar]
- Yamakuchi, M.; Lowenstein, C.J. MiR-34, SIRT1 and p53: The feedback loop. Cell Cycle 2009, 8, 712–715. [Google Scholar] [CrossRef]
- Xu, D.M.; Li, H.; Zhao, Y.; Wang, C.S. Downregulation of miR-34a attenuates myocardial ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Int. J. Clin. Exp. Pathol. 2017, 10, 3865–3875. [Google Scholar]
- Yang, Y.; Cheng, H.W.; Qiu, Y.; Dupee, D.; Noonan, M.; Lin, Y.D.; Fisch, S.; Unno, K.; Sereti, K.I.; Liao, R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015, 117, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Luscher, T.F.; Cosentino, F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur. Heart J. 2016, 37, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.B.; Li, Z.Y.; Li, H.; Fan, X.Q.; Liu, H.G.; Dong, X.M.; Jia, W.Y. Inhibitive effects of microRNA-34a on protecting against ischemia-reperfusion injury of vital organs in hemorrhagic shock pregnant mice. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1812–1818. [Google Scholar] [PubMed]
- Ito, T.; Yagi, S.; Yamakuchi, M. MicroRNA-34a regulation of endothelial senescence. Biochem. Biophys. Res. Commun. 2010, 398, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Vasa-Nicotera, M.; Ippoliti, A.; Novelli, G.; Melino, G.; et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 2009, 120, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Khanna, A.; Li, N.; Wang, E. Circulatory miR-34a as an RNA-based, noninvasive biomarker for brain aging. Aging (Albany NY) 2011, 3, 985. [Google Scholar] [CrossRef]
- Zhao, T.; Li, J.; Chen, A.F. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E110–E116. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.; He, Y.; Melendez, A.; Feng, Z.; Hong, Q.; Bai, X.; Li, Q.; Cai, G.; Wang, J.; et al. MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age (Dordr) 2013, 35, 11–22. [Google Scholar] [CrossRef]
- Xu, P.; Damschroder, D.; Zhang, M.; Ryall, K.A.; Adler, P.N.; Saucerman, J.J.; Wessells, R.J.; Yan, Z. Atg2, Atg9 and Atg18 in mitochondrial integrity, cardiac function and healthspan in Drosophila. J. Mol. Cell Cardiol. 2018, 127, 116–124. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Zhang, B.; Shi, Y.; Cui, L.; Zhao, X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc. Pathol. 2015, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rohailla, S.; Gelber, N.; Rutka, J.; Sabah, N.; Gladstone, R.A.; Wei, C.; Hu, P.; Kharbanda, R.K.; Redington, A.N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res. Cardiol. 2014, 109, 423. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, M.; Qiao, Y.; Wang, Y.; Xiong, W.; Yue, W. Suppression of microRNA-144-3p attenuates oxygen-glucose deprivation/reoxygenation-induced neuronal injury by promoting Brg1/Nrf2/ARE signaling. J. Biochem. Mol. Toxicol. 2018, 32, e22044. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, S.X.; He, Q.; Zhang, H.; Friedberg, D.; Wang, F.; Redington, A.N. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res. Cardiol. 2018, 113, 36. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Kusakari, Y.; Xiao, C.Y.; Inouye, B.T.; Takahashi, M.; Scherrer-Crosbie, M.; Rosenzweig, A.; Hara, K.; Matsui, T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H75–H85. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, Z.; Wang, L. Small interference RNA against PTP-1B reduces hypoxia/reoxygenation induced apoptosis of rat cardiomyocytes. Apoptosis 2008, 13, 383–393. [Google Scholar] [CrossRef]
- Shi, Y.F.; Liu, N.; Li, Y.X.; Song, C.L.; Song, X.J.; Zhao, Z.; Liu, B. Insulin protects H9c2 rat cardiomyoblast cells against hydrogen peroxide-induced injury through upregulation of microRNA-210. Free Radic. Res. 2015, 49, 1147–1155. [Google Scholar] [CrossRef]
- Diao, H.; Liu, B.; Shi, Y.; Song, C.; Guo, Z.; Liu, N.; Song, X.; Lu, Y.; Lin, X.; Li, Z. MicroRNA-210 alleviates oxidative stress-associated cardiomyocyte apoptosis by regulating BNIP3. Biosci. Biotechnol. Biochem. 2017, 81, 1712–1720. [Google Scholar] [CrossRef]
- Feng, M.; Li, Z.; Wang, D.; Wang, F.; Wang, C.; Wang, C.; Ding, F. MicroRNA-210 aggravates hypoxia-induced injury in cardiomyocyte H9c2 cells by targeting CXCR4. Biomed. Pharmacother. 2018, 102, 981–987. [Google Scholar] [CrossRef]
- Mahalakshmi, A.; Kurian, G.A. Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radic. Biol. Med. 2018, 118, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Roy, S.; Banerjee, J.; Hussain, S.R.; Khanna, S.; Meenakshisundaram, G.; Kuppusamy, P.; Friedman, A.; Sen, C.K. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc. Natl. Acad. Sci. USA 2010, 107, 6976–6981. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.E.; Camps, C.; Buffa, F.M.; Patiar, S.; Winter, S.C.; Betts, G.; Homer, J.; Corbridge, R.; Cox, G.; West, C.M.; et al. hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer 2010, 116, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Huang, H.; Zhang, M.; Chen, M.; Huang, H.; Huang, F.; Zhou, S. HMGB1 induces apoptosis and EMT in association with increased autophagy following H/R injury in cardiomyocytes. Int. J. Mol. Med. 2016, 37, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xiao, W.; Zeng, Y.; Liu, M.H.; Li, G.H.; Tang, Z.H.; Qu, S.L.; Hao, Y.M.; Yuan, H.Q.; Jiang, Z.S. Fibroblast growth factor-21 alleviates hypoxia/reoxygenation injury in H9c2 cardiomyocytes by promoting autophagic flux. Int. J. Mol. Med. 2019, 43, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lew, K.S.; Chen, Q.; Richards, A.M.; Wang, P. Human mesenchymal stem cell-derived exosomes reduce ischemia/reperfusion injury by the inhibitions of apoptosis and autophagy. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Ma, M.; Zheng, Y.; Pan, Y.; Lin, X. mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Xu, T.X.; Zhao, S.Z.; Dong, M.; Yu, X.R. Hypoxia responsive miR-210 promotes cell survival and autophagy of endometriotic cells in hypoxia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 399–406. [Google Scholar]
- Singhal, A.K.; Symons, J.D.; Boudina, S.; Jaishy, B.; Shiu, Y.T. Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc. Dis. Prev. 2010, 7, 1–14. [Google Scholar] [CrossRef]
- Brutsaert, D.L. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 2003, 83, 59–115. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; Colombo, S.L.; Godfrey, A.; Moncada, S. Mitochondria as signaling organelles in the vascular endothelium. Proc. Natl. Acad. Sci. USA 2006, 103, 5379–5384. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.R.; Li, J.; Gong, J.Y.; Kuang, F.; Liu, J.Y.; Zhang, Y.S.; Ma, Q.L.; Song, C.J.; Truax, A.D.; Gao, F.; et al. MicroRNA-141 regulates the expression level of ICAM-1 on endothelium to decrease myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1303–H1313. [Google Scholar] [CrossRef]
- Saini, H.K.; Xu, Y.J.; Zhang, M.; Liu, P.P.; Kirshenbaum, L.A.; Dhalla, N.S. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp. Clin. Cardiol. 2005, 10, 213–222. [Google Scholar] [PubMed]
- Maekawa, N.; Wada, H.; Kanda, T.; Niwa, T.; Yamada, Y.; Saito, K.; Fujiwara, H.; Sekikawa, K.; Seishima, M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J. Am. Coll. Cardiol. 2002, 39, 1229–1235. [Google Scholar] [CrossRef]
- Bellisarii, F.L.; Gallina, S.; De Caterina, R. Tumor necrosis factor-alpha and cardiovascular diseases. Ital. Heart J. 2001, 2, 408–417. [Google Scholar]
- Dhote-Burger, P.; Vuilleminot, A.; Lecompte, T.; Pasquier, C.; Bara, L.; Julia, P.; Chardigny, C.; Fabiani, J.N. Neutrophil degranulation related to the reperfusion of ischemic human heart during cardiopulmonary bypass. J. Cardiovasc. Pharmacol. 1995, 25 (Suppl. 2), S124–S129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Doerschuk, C.M. Neutrophil-induced changes in the biomechanical properties of endothelial cells: Roles of ICAM-1 and reactive oxygen species. J. Immunol. 2000, 164, 6487–6494. [Google Scholar] [CrossRef]
- Saito, S.; Thuc, L.C.; Teshima, Y.; Nakada, C.; Nishio, S.; Kondo, H.; Fukui, A.; Abe, I.; Ebata, Y.; Saikawa, T.; et al. Glucose Fluctuations Aggravate Cardiac Susceptibility to Ischemia/Reperfusion Injury by Modulating MicroRNAs Expression. Circ. J. 2016, 80, 186–195. [Google Scholar] [CrossRef]
- Martinesi, M.; Treves, C.; D’albasio, G.; Bagnoli, S.; Bonanomi, A.G.; Stio, M. Vitamin D derivatives induce apoptosis and downregulate ICAM-1 levels in peripheral blood mononuclear cells of inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 597–604. [Google Scholar] [CrossRef]
- Li, X.-Y.; Wang, S.-S.; Han, Z.; Han, F.; Chang, Y.-P.; Yang, Y.; Xue, M.; Sun, B.; Chen, L.-M. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol. Ther. Nucl. Acids 2017, 9, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hou, Y.; Liu, Y.; Zheng, J. LncRNA SNHG15 contributes to proliferation, invasion and autophagy in osteosarcoma cells by sponging miR-141. J. Biomed. Sci. 2017, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Xue, J.; Yang, Z.; Shi, Y.; Shi, Y.; Lou, G.; Wu, S.; Qi, J.; Liu, W.; et al. MicroRNA-141 Targets Sirt1 and Inhibits Autophagy to Reduce HBV Replication. Cell. Physiol. Biochem. 2017, 41, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, L.; Zhu, S.; Li, X.; Li, Z.; Yu, C.; Yu, X. Regulation of autophagy by systemic admission of microrna-141 to target hmgb1 in l-arginine-induced acute pancreatitis in vivo. Pancreatology 2016, 16, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Preusse, M.; Theis, F.J.; Mueller, N.S. Mitalos v2: Analyzing tissue specific microrna function. PLoS ONE 2016, 11, e0151771. [Google Scholar] [CrossRef] [PubMed]
- Eisenhardt, S.U.; Weiss, J.B.; Smolka, C.; Maxeiner, J.; Pankratz, F.; Bemtgen, X.; Kustermann, M.; Thiele, J.R.; Schmidt, Y.; Bjoern Stark, G.; et al. Microrna-155 aggravates ischemia-reperfusion injury by modulation of inflammatory cell recruitment and the respiratory oxidative burst. Basic Res. Cardiol. 2015, 110, 32. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Ma, Y.L.; Liang, W.; Li, H.H.; Ma, Z.J.; Yu, X.; Liao, Y.H. Microrna-155 modulates treg and th17 cells differentiation and th17 cell function by targeting socs1. PLoS ONE 2012, 7, e46082. [Google Scholar] [CrossRef]
- Kwon, D.N.; Chang, B.S.; Kim, J.H. Microrna dysregulation in liver and pancreas of cmp-neu5ac hydroxylase null mice disrupts insulin/pi3k-akt signaling. Biomed. Res. Int. 2014, 2014, 236385. [Google Scholar] [CrossRef]
- Jia, C.; Chen, H.; Wei, M.; Chen, X.; Zhang, Y.; Cao, L.; Yuan, P.; Wang, F.; Yang, G.; Ma, J. Gold nanoparticle-based mir155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int. J. Nanomed. 2017, 12, 4963–4979. [Google Scholar] [CrossRef]
- Ye, J.; Kang, Y.; Sun, X.; Ni, P.; Wu, M.; Lu, S. Microrna-155 inhibition promoted wound healing in diabetic rats. Int. J. Low. Extrem. Wounds 2017, 16, 74–84. [Google Scholar] [CrossRef]
- Kishore, R.; Verma, S.K.; Mackie, A.R.; Vaughan, E.E.; Abramova, T.V.; Aiko, I.; Krishnamurthy, P. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac mirna-155 modulates fibrotic response in diabetic hearts. PLoS ONE 2013, 8, e60161. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huang, H.; Xie, Q.; Wang, Z.; Fan, Y.; Kong, B.; Huang, D.; Xiao, Y. Mir-155 knockout in fibroblasts improves cardiac remodeling by targeting tumor protein p53-inducible nuclear protein 1. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yan, X.; Yan, L.; Hu, F.; Ma, W.; Wang, Y.; Lu, S.; Zeng, Q.; Wang, Z. Inhibition of microrna155 ameliorates cardiac fibrosis in the process of angiotensin iiinduced cardiac remodeling. Mol. Med. Rep. 2017, 16, 7287–7296. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Zeng, D.Y.; Li, R.T.; Pang, R.P.; Yang, H.; Hu, Y.L.; Zhang, Q.; Jiang, Y.; Huang, L.Y.; Tang, Y.B.; et al. Essential role of microrna-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 2012, 60, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Fan, W.D.; Fang, R.; Wu, G.F. Regulation of microrna-155 in endothelial inflammation by targeting nuclear factor (nf)-kappab p65. J. Cell. Biochem. 2014, 115, 1928–1936. [Google Scholar] [PubMed]
- Tian, F.J.; An, L.N.; Wang, G.K.; Zhu, J.Q.; Li, Q.; Zhang, Y.Y.; Zeng, A.; Zou, J.; Zhu, R.F.; Han, X.S.; et al. Elevated microrna-155 promotes foam cell formation by targeting hbp1 in atherogenesis. Cardiovasc. Res. 2014, 103, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Li, X.; Tang, Z.; Wang, X.; Zhong, M.; Suo, Q.; Zhang, Y.; Lv, K. Silencing microrna-155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci. Rep. 2016, 6, 22613. [Google Scholar] [CrossRef]
- Cao, R.Y.; Li, Q.; Miao, Y.; Zhang, Y.; Yuan, W.; Fan, L.; Liu, G.; Mi, Q.; Yang, J. The emerging role of microrna-155 in cardiovascular diseases. Biomed. Res. Int. 2016, 2016, 9869208. [Google Scholar] [CrossRef]
- Chen, H.; Gao, L.; Yang, M.; Zhang, L.; He, F.L.; Shi, Y.K.; Pan, X.H.; Wang, H. Microrna-155 affects oxidative damage through regulating autophagy in endothelial cells. Oncol. Lett. 2019, 17, 2237–2243. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, P.; Yang, J.; Liu, X.; Dong, S.; Wang, X.; Chun, B.; Zhuang, J.; Zhang, C. Ischaemic preconditioning-regulated mir-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target pdcd4. Cardiovasc. Res. 2010, 87, 431–439. [Google Scholar] [CrossRef]
- Wang, X.; Ha, T.; Liu, L.; Zou, J.; Zhang, X.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. Increased expression of microrna-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013, 97, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Olson, J.M.; Paterson, M.; Yan, Y.; Zaja, I.; Liu, Y.; Riess, M.L.; Kersten, J.R.; Liang, M.; Warltier, D.C.; et al. Microrna-21 mediates isoflurane-induced cardioprotection against ischemia-reperfusion injury via akt/nitric oxide synthase/mitochondrial permeability transition pore pathway. Anesthesiology 2015, 123, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Bai, J.; Zhang, W.; Luo, H.; Zhang, X.; Liu, D.; Qiao, C. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac mirna21 expression, akt and the bcl2/bax pathway. Mol. Med. Rep. 2016, 14, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.H.; Lee, S.Y.; Lee, C.Y.; Kim, R.; Kim, P.; Oh, S.; Lee, H.; Lee, M.Y.; Kim, J.; Kim, L.K.; et al. Exogenous mirna-146a enhances the therapeutic efficacy of human mesenchymal stem cells by increasing vascular endothelial growth factor secretion in the ischemia/reperfusion-injured heart. J. Vasc. Res. 2017, 54, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhou, Y.; Zhai, H.; Li, L.; Sun, L.; Li, Y. The role of microrna-146a in regulating the expression of irak1 in cerebral ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2018, 96, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.M.; Williams, A.E.; Tsitsiou, E.; Larner-Svensson, H.M.; Lindsay, M.A. Divergent intracellular pathways regulate interleukin-1beta-induced mir-146a and mir-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett. 2009, 583, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. Microrna-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting kbtbd7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. Microrna-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene pdcd4. J. Mol. Cell. Cardiol. 2009, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Brudecki, L.; Ferguson, D.A.; McCall, C.E.; El Gazzar, M. Microrna-146a and rbm4 form a negative feed-forward loop that disrupts cytokine mrna translation following tlr4 responses in human thp-1 monocytes. Immunol. Cell. Biol. 2013, 91, 532–540. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, M.; He, X.; Wang, B.; Li, Y.; Guo, X. Overexpression of microrna-146 protects against oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis by inhibiting the nf-kappab/tnf-alpha signaling pathway. Mol. Med. Rep. 2018, 17, 1913–1918. [Google Scholar] [PubMed]
- Rong, Y.; Bao, W.; Shan, Z.; Liu, J.; Yu, X.; Xia, S.; Gao, H.; Wang, X.; Yao, P.; Hu, F.B.; et al. Increased microrna-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE 2013, 8, e73272. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.; Venugopal, B.; Sekar, P.; Ramalingam, K. Role of microrna 21 in diabetes and associated/related diseases. Gene 2016, 582, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.Y.; Lee, T.P.; Chen, C.Y.; Chiu, Y.H.; Lin, Y.C.; Lee, L.S.; Li, W.C. Circulating microrna as a diagnostic marker in populations with type 2 diabetes mellitus and diabetic complications. J. Chin. Med. Assoc. 2015, 78, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wan, L.; Fan, Y.; Wang, K.; Bu, L.; Huang, T.; Cheng, Z.; Shen, B. Ischemic postconditioning-mediated mirna-21 protects against cardiac ischemia/reperfusion injury via pten/akt pathway. PLoS ONE 2013, 8, e75872. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Das, A.; Mezzaroma, E.; Chau, V.Q.; Marchetti, C.; Durrant, D.; Samidurai, A.; Van Tassell, B.W.; Yin, C.; Ockaili, R.A.; et al. Induction of microrna-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ. Cardiovasc. Genet. 2014, 7, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qiu, F.; Zhou, K.; Matlock, H.G.; Takahashi, Y.; Rajala, R.V.S.; Yang, Y.; Moran, E.; Ma, J.X. Pathogenic role of microrna-21 in diabetic retinopathy through downregulation of pparalpha. Diabetes 2017, 66, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, S.; McArthur, K.; Wu, Y.; Sen, S.; Ding, Q.; Feldman, R.D.; Chakrabarti, S. Mir-146a-mediated extracellular matrix protein production in chronic diabetes complications. Diabetes 2011, 60, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chu, A.; Feng, Y.; Chen, L.; Shao, Y.; Luo, Q.; Deng, X.; Wu, M.; Shi, X.; Chen, Y. Microrna-146a: A comprehensive indicator of inflammation and oxidative stress status induced in the brain of chronic t2dm rats. Front. Pharmacol. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Capdevila-Busquets, E.; Botteri, G.; Davidson, M.M.; Rodriguez, C.; Martinez-Gonzalez, J.; Vidal, F.; Barroso, E.; Chan, T.O.; Feldman, A.M.; et al. Mir-146a targets fos expression in human cardiac cells. Dis. Model. Mech. 2015, 8, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, S.; Gordon, A.D.; Chakrabarti, S. Mir-146a mediates inflammatory changes and fibrosis in the heart in diabetes. J. Mol. Cell. Cardiol. 2017, 105, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, D.; Scott, G.K.; Schokrpur, S.; Patil, C.K.; Orjalo, A.V.; Rodier, F.; Lithgow, G.J.; Campisi, J. Micrornas mir-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009, 1, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Lazzarini, R.; Recchioni, R.; Marcheselli, F.; Rippo, M.R.; Di Nuzzo, S.; Albertini, M.C.; Graciotti, L.; Babini, L.; Mariotti, S. Mir-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age 2013, 35, 1157–1172. [Google Scholar] [CrossRef]
- Chassin, C.; Hempel, C.; Stockinger, S.; Dupont, A.; Kubler, J.F.; Wedemeyer, J.; Vandewalle, A.; Hornef, M.W. Microrna-146a-mediated downregulation of irak1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol. Med. 2012, 4, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, S.; Kong, F.; Cai, X.; Ye, B.; Shan, P.; Huang, W. Microrna-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in h9c2 cells via the akt/mtor pathway. J. Cell. Mol. Med. 2017, 21, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Z.; Liang, B.; Li, L.; Liu, S.; Tan, W.; Long, J.; Tang, F.; Chu, C.; Yang, J. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through pi3k/akt signaling pathway. Endocr. J. 2018, EJ17-0445. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.M.; Schauerte, C.; Hubner, A.; Kolling, M.; Martino, F.; Scherf, K.; Batkai, S.; Zimmer, K.; Foinquinos, A.; Kaucsar, T.; et al. Osteopontin is indispensible for ap1-mediated angiotensin ii-related mir-21 transcription during cardiac fibrosis. Eur. Heart J. 2015, 36, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Shi, P.; Ge, J.J. Mir-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via cadm1/stat3 pathway. BMC Cardiovasc. Disord. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. Microrna-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Xu, X.; Kriegel, A.J.; Jiao, X.; Liu, H.; Bai, X.; Olson, J.; Liang, M.; Ding, X. Mir-21 in ischemia/reperfusion injury: A double-edged sword? Physiol. Genomics 2014, 46, 789–797. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, W.; Huang, G.; Huang, C.; Zhu, X.; Su, G.; Xu, J. Troxerutin attenuates myocardial cell apoptosis following myocardial ischemia-reperfusion injury through inhibition of mir-146a-5p expression. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- D’Agostino, M.; Martino, F.; Sileno, S.; Barilla, F.; Beji, S.; Marchetti, L.; Gangi, F.M.; Persico, L.; Picozza, M.; Montali, A.; et al. Circulating mir-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with mir-33a/b levels: Implication of a zeb1-dependent mechanism. Clin. Sci. (Lond) 2017, 131, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Belgardt, B.F.; Ahmed, K.; Spranger, M.; Latreille, M.; Denzler, R.; Kondratiuk, N.; von Meyenn, F.; Villena, F.N.; Herrmanns, K.; Bosco, D.; et al. The microrna-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat. Med. 2015, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Ciarapica, R.; Capogrossi, M.C. The emerging role of mir-200 family in cardiovascular diseases. Circ. Res. 2017, 120, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. Mir-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via zeb1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative stress-induced mir-200c disrupts the regulatory loop among sirt1, foxo1, and enos. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.A.; Jin, W.; Villeneuve, L.; Wang, M.; Lanting, L.; Todorov, I.; Kato, M.; Natarajan, R. Pro-inflammatory role of microrna-200 in vascular smooth muscle cells from diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Qu, D.; Wang, L.; Luo, J.Y.; Lau, C.W.; Liu, P.; Gao, Z.; Tipoe, G.L.; Lee, H.K.; et al. Inhibition of mir-200c restores endothelial function in diabetic mice through suppression of cox-2. Diabetes 2016, 65, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Yoon, H.J.; Jeon, D.; Kang, K.M.; Park, K.H.; Bae, E.K.; Kim, M.; Lee, S.K.; et al. Micrornas induced during ischemic preconditioning. Stroke 2010, 41, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Cao, Y.; Chen, S.; Chu, X.; Chu, Y.; Chakrabarti, S. Mir-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes 2016, 65, 768–779. [Google Scholar] [CrossRef]
- Dantas da Costa, E.S.M.E.; Polina, E.R.; Crispim, D.; Sbruzzi, R.C.; Lavinsky, D.; Mallmann, F.; Martinelli, N.C.; Canani, L.H.; Dos Santos, K.G. Plasma levels of mir-29b and mir-200b in type 2 diabetic retinopathy. J. Cell. Mol. Med. 2019, 23, 1280–1287. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Xu, R.; Nie, Y.; Cao, X.; Meng, J.; Xu, X.; Hu, S.; Zheng, Z. Microrna-24 regulates cardiac fibrosis after myocardial infarction. J. Cell. Mol. Med. 2012, 16, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Van Laake, L.W.; Huang, Y.; Liu, S.; Wendland, M.F.; Srivastava, D. Mir-24 inhibits apoptosis and represses bim in mouse cardiomyocytes. J. Exp. Med. 2011, 208, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, G.C. Role of micrornas in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012, 94, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Shantikumar, S.; Caporali, A.; Emanueli, C. Role of micrornas in diabetes and its cardiovascular complications. Cardiovasc. Res. 2012, 93, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Wang, H.; Przybilla, D.; Franklin, B.S.; Dolf, A.; Pfeifer, P.; Schmitz, T.; Flender, A.; Endl, E.; Nickenig, G.; et al. Vascular endothelial microparticles-incorporated micrornas are altered in patients with diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Thandavarayan, R.A.; Joladarashi, D.; Jeyabal, P.; Krishnamurthy, S.; Bhimaraj, A.; Youker, K.A.; Krishnamurthy, P. Microrna-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci. Rep. 2016, 6, 36207. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Bei, Y.; Kong, X.; Liu, X.; Lei, Z.; Xu, T.; Wang, H.; Xuan, Q.; Chen, P.; Xu, J.; et al. Mir-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 2017, 7, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chen, M.; Rao, Z.; Qiu, Y.; Liu, J.; Jiang, Y.; Huang, Z.; Wang, X.; Lin, T. Mir-17-92 ameliorates renal ischemia reperfusion injury. Kaohsiung J. Med. Sci. 2018, 34, 263–273. [Google Scholar] [CrossRef]
- Hao, J.; Wei, Q.; Mei, S.; Li, L.; Su, Y.; Mei, C.; Dong, Z. Induction of microrna-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int. 2017, 91, 106–118. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Wang, Z.; Wang, T.; Yu, Y.; He, J.; Zhang, H.; Yang, T.; Shen, Z. Microrna-17 regulates autophagy to promote hepatic ischemia/reperfusion injury via suppression of signal transductions and activation of transcription-3 expression. Liver Transplant. 2016, 22, 1697–1709. [Google Scholar] [CrossRef]
- Zhu, H.J.; Wang, D.G.; Yan, J.; Xu, J. Up-regulation of microrna-135a protects against myocardial ischemia/reperfusion injury by decreasing txnip expression in diabetic mice. Am. J. Translant. Res. 2015, 7, 2661–2671. [Google Scholar] [PubMed]
- Cheng, Y.; Sun, T.; Yin, C.; Wang, S.; Li, Z.; Tao, Y.; Zhang, J.; Li, Z.; Zhang, H. Downregulation of pten by sodium orthovanadate protects the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment. J. Cell. Biochem. 2019, 120, 3709–3715. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.L.; Fang, H.C.; Zhao, H.L.; Li, X.L.; Luo, Y.; Wu, B.Q.; Fu, M.J.; Liu, W.; Liang, J.J.; Chen, X.H. The role of microrna-1 targeting of mapk3 in myocardial ischemia-reperfusion injury in rats undergoing sevoflurane preconditioning via the pi3k/akt pathway. Am. J. Physiol. Cell. Physiol. 2018, 315, C380–C388. [Google Scholar] [CrossRef] [PubMed]
- Zaccagnini, G.; Maimone, B.; Fuschi, P.; Maselli, D.; Spinetti, G.; Gaetano, C.; Martelli, F. Overexpression of mir-210 and its significance in ischemic tissue damage. Sci. Rep. 2017, 7, 9563. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.F.; Li, D.C.; Shen, Y.Q.; Wang, C.L.; Zhang, D.Y.; Shang, A.Q.; Hu, T. Mir-146b protects cardiomyocytes injury in myocardial ischemia/reperfusion by targeting smad4. Am. J. Translant. Res. 2017, 9, 656–663. [Google Scholar]
- Zuo, Y.; Wang, Y.; Hu, H.; Cui, W. Atorvastatin protects myocardium against ischemia-reperfusion injury through inhibiting mir-199a-5p. Cell. Physiol. Biochem. 2016, 39, 1021–1030. [Google Scholar] [CrossRef]
- Park, K.M.; Teoh, J.P.; Wang, Y.; Broskova, Z.; Bayoumi, A.S.; Tang, Y.; Su, H.; Weintraub, N.L.; Kim, I.M. Carvedilol-responsive micrornas, mir-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H371–H383. [Google Scholar] [CrossRef]
- Zeng, X.C.; Li, L.; Wen, H.; Bi, Q. Microrna-128 inhibition attenuates myocardial ischemia/reperfusion injury-induced cardiomyocyte apoptosis by the targeted activation of peroxisome proliferator-activated receptor gamma. Mol. Med. Rep. 2016, 14, 129–136. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, X.; Ren, J.; Li, X.; Gao, X.; Lu, C.; Zhang, Y.; Sun, H.; Wang, Y.; Wang, H.; et al. Mir-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS ONE 2012, 7, e50515. [Google Scholar] [CrossRef]
- Ye, Y.; Hu, Z.; Lin, Y.; Zhang, C.; Perez-Polo, J.R. Downregulation of microrna-29 by antisense inhibitors and a ppar-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2010, 87, 535–544. [Google Scholar] [CrossRef]
- Hu, S.; Huang, M.; Li, Z.; Jia, F.; Ghosh, Z.; Lijkwan, M.A.; Fasanaro, P.; Sun, N.; Wang, X.; Martelli, F. Microrna-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010, 122, S124–S131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Ren, X.-P.; Chen, J.; Liu, H.; Yang, J.; Medvedovic, M.; Hu, Z.; Fan, G.-C. Microrna-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injuryclinical perspective. Circulation 2010, 122, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.-C. Microrna-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Niu, X.; Hu, J.; Xing, H.; Sun, M.; Wang, J.; Jian, Q.; Yang, H. After myocardial ischemia-reperfusion, mir-29a, and let7 could affect apoptosis through regulating igf-1. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]




| miRNA | Function | Level in Diabetes |
|---|---|---|
| miR-34 |
| increased |
| miR-144 |
| decreased |
| miR-210 |
| increased |
| miR-141 |
| increased |
| miR-155 |
| increased |
| miR-21 |
| increased |
| miR-146 |
| increased |
| miR-200b |
| decreased |
| miR-200c |
| increased |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehaini, H.; Awada, H.; El-Yazbi, A.; Zouein, F.A.; Issa, K.; Eid, A.A.; Ibrahim, M.; Badran, A.; Baydoun, E.; Pintus, G.; et al. MicroRNAs as Potential Pharmaco-Targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells 2019, 8, 152. https://doi.org/10.3390/cells8020152
Dehaini H, Awada H, El-Yazbi A, Zouein FA, Issa K, Eid AA, Ibrahim M, Badran A, Baydoun E, Pintus G, et al. MicroRNAs as Potential Pharmaco-Targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells. 2019; 8(2):152. https://doi.org/10.3390/cells8020152
Chicago/Turabian StyleDehaini, Hassan, Hussein Awada, Ahmed El-Yazbi, Fouad A. Zouein, Khodr Issa, Assaad A. Eid, Maryam Ibrahim, Adnan Badran, Elias Baydoun, Gianfranco Pintus, and et al. 2019. "MicroRNAs as Potential Pharmaco-Targets in Ischemia-Reperfusion Injury Compounded by Diabetes" Cells 8, no. 2: 152. https://doi.org/10.3390/cells8020152