Potential Utility of Urinary Follistatin as a Non-Invasive Indicator of Acute Tubular Damage in Patients with Acute Kidney Injury
Abstract
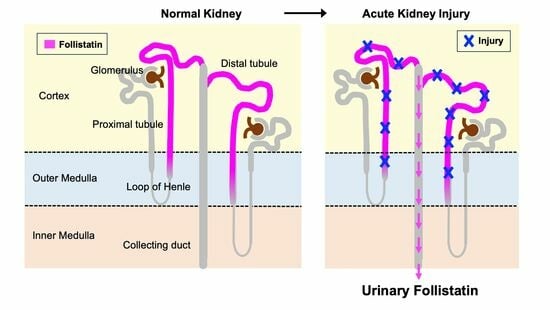
:1. Introduction
2. Materials and Methods
2.1. Setting and Patients
2.2. Sample and Data Collection
2.3. Immunohistochemical Analysis
2.4. ELISA
2.5. Statistical Analysis
3. Results
3.1. Localization of Follistatin in Normal Human Kidney
3.2. Baseline Characteristics of Patients
3.3. Significant Increase in Follistatin in the Urine of AKI Patients
3.4. Urinary Follistatin Is Associated with the Severity and Prognosis of AKI
3.5. Correlation between Urinary Follistatin and Clinical Parameters
3.6. Time Course of Changes in Urinary Follistatin and Other AKI Biomarkers in an AKI Patient
3.7. Urinary Follistatin in Patients with Different Causes of AKI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-term risk of adverse outcomes after acute kidney injury: A systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Chawla, L.S.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. Outcomes following diagnosis of acute renal failure in US veterans: Focus on acute tubular necrosis. Kidney Int. 2009, 76, 1089–1097. [Google Scholar] [CrossRef]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordoñez, J.D.; Hsu, C.-y. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009, 76, 893–899. [Google Scholar] [CrossRef]
- Wald, R.; Quinn, R.R.; Luo, J.; Li, P.; Scales, D.C.; Mamdani, M.M.; Ray, J.G.; Group, U.o.T.A.K.I.R. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA J. Am. Med. Assoc. 2009, 302, 1179–1185. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Coca, S.G.; Wang, Y.; Masoudi, F.A.; Krumholz, H.M. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch. Intern. Med. 2008, 168, 987–995. [Google Scholar] [CrossRef]
- Chawla, L.S. Acute kidney injury leading to chronic kidney disease and long-term outcomes of acute kidney injury: The best opportunity to mitigate acute kidney injury? Controv. Acute Kidney Inj. 2011, 174, 182–190. [Google Scholar]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Parikh, C.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006, 70, 199–203. [Google Scholar] [CrossRef]
- Portilla, D.; Dent, C.; Sugaya, T.; Nagothu, K.; Kundi, I.; Moore, P.; Noiri, E.; Devarajan, P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008, 73, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Koyner, J.L.; Shaw, A.D.; Chawla, L.S.; Hoste, E.A.; Bihorac, A.; Kashani, K.; Haase, M.; Shi, J.; Kellum, J.A. Tissue inhibitor metalloproteinase-2 (TIMP-2)⋅ IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J. Am. Soc. Nephrol. 2015, 26, 1747–1754. [Google Scholar] [CrossRef]
- Esch, F.S.; Shimasaki, S.; Mercado, M.; Cooksey, K.; Ling, N.; Ying, S.; Ueno, N.; Guillemin, R. Structural characterization of follistatin: A novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol. Endocrinol. 1987, 1, 849–855. [Google Scholar] [CrossRef]
- Nakamura, T.; Takio, K.; Eto, Y.; Shibai, H.; Titani, K.; Sugino, H. Activin-binding protein from rat ovary is follistatin. Science 1990, 247, 836–838. [Google Scholar] [CrossRef]
- Maeshima, A.; Zhang, Y.-q.; Nojima, Y.; Naruse, T.; Kojima, I. Involvement of the activin-follistatin system in tubular regeneration after renal ischemia in rats. J. Am. Soc. Nephrol. 2001, 12, 1685–1695. [Google Scholar] [CrossRef]
- Yamashita, S.; Maeshima, A.; Kojima, I.; Nojima, Y. Activin A is a potent activator of renal interstitial fibroblasts. J. Am. Soc. Nephrol. 2004, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Koken, E.; Oyar, E.O.; Uyanikgil, Y.; Pazarlar, B.A.; Bilister, C.; Aksun, S.; Yigitturk, G.; Koken, E.C. Exogenous follistatin administration ameliorates cisplatin-induced acute kidney injury through anti-inflammation and anti-apoptosis effects. Bratisl. Lek. Listy 2020, 121, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Gava, A.L.; Zhang, D.; Gao, B.; Krepinsky, J.C. Follistatin Protects Against Glomerular Mesangial Cell Apoptosis and Oxidative Stress to Ameliorate Chronic Kidney Disease. Antioxid. Redox Signal 2019, 31, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Nakasatomi, M.; Takei, Y.; Ikeuchi, H.; Sakairi, T.; Kaneko, Y.; Hiromura, K.; Nojima, Y.; Maeshima, A. Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury. Sci. Rep. 2018, 8, 5176. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, I.; Maeshima, A.; Nagata, D. Urinary Activin A: A Novel Biomarker for Human Acute Kidney Injury. Diagnostics 2022, 12, 661. [Google Scholar] [CrossRef]
- Palevsky, P.M.; Liu, K.D.; Brophy, P.D.; Chawla, L.S.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, I.; Takayanagi, K.; Hasegawa, H.; Maeshima, A. Tubule-Derived Follistatin Is Increased in the Urine of Rats with Renal Ischemia and Reflects the Severity of Acute Tubular Damage. Cells 2023, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, K.; Ogawa, K.; Hayashi, Y.; Nakamura, T.; Titani, K.; Sugino, H. Immunohistochemical localization of follistatin in rat tissues. Endocrinol. Jpn. 1991, 38, 383–391. [Google Scholar] [CrossRef]
- Miyamoto, T.; Carrero, J.J.; Qureshi, A.R.; Anderstam, B.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Stenvinkel, P. Circulating follistatin in patients with chronic kidney disease: Implications for muscle strength, bone mineral density, inflammation, and survival. Clin. J. Am. Soc. Nephrol. 2011, 6, 1001–1008. [Google Scholar] [CrossRef]
- Hansen, J.S.; Plomgaard, P. Circulating follistatin in relation to energy metabolism. Mol. Cell Endocrinol. 2016, 433, 87–93. [Google Scholar] [CrossRef]
- Näf, S.; Escote, X.; Ballesteros, M.; Yañez, R.E.; Simon-Muela, I.; Gil, P.; Albaiges, G.; Vendrell, J.; Megia, A. Serum activin A and follistatin levels in gestational diabetes and the association of the Activin A-Follistatin system with anthropometric parameters in offspring. PLoS ONE 2014, 9, e92175. [Google Scholar] [CrossRef]
- Han, X.; Møller, L.L.V.; De Groote, E.; Bojsen-Møller, K.N.; Davey, J.; Henríquez-Olguin, C.; Li, Z.; Knudsen, J.R.; Jensen, T.E.; Madsbad, S. Mechanisms involved in follistatin-induced hypertrophy and increased insulin action in skeletal muscle. J. Cachexia Sarcopenia Muscle 2019, 10, 1241–1257. [Google Scholar] [CrossRef]
- Panagiotou, G.; Ghaly, W.; Upadhyay, J.; Pazaitou-Panayiotou, K.; Mantzoros, C.S. Serum follistatin is increased in thyroid cancer and is associated with Adverse tumor characteristics in humans. J. Clin. Endocrinol. Metab. 2021, 106, e2137–e2150. [Google Scholar] [CrossRef]
- Tomoda, T.; Nouso, K.; Miyahara, K.; Kobayashi, S.; Kinugasa, H.; Toyosawa, J.; Hagihara, H.; Kuwaki, K.; Onishi, H.; Nakamura, S. Prognotic impact of serum follistatin in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2013, 28, 1391–1396. [Google Scholar] [CrossRef]
- Zhang, P.; Ruan, Y.; Xiao, J.; Chen, F.; Zhang, X. Association of serum follistatin levels with histological types and progression of tumor in human lung cancer. Cancer Cell Int. 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Mao, C.; Li, J.; Han, F.; Yang, P. Effects of the activin A–follistatin system on myocardial cell apoptosis through the endoplasmic reticulum stress pathway in heart failure. Int. J. Mol. Sci. 2017, 18, 374. [Google Scholar] [CrossRef]
- Chen, Y.; Rothnie, C.; Spring, D.; Verrier, E.; Venardos, K.; Kaye, D.; Phillips, D.J.; Hedger, M.P.; Smith, J.A. Regulation and actions of activin A and follistatin in myocardial ischaemia–reperfusion injury. Cytokine 2014, 69, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Yoshioka, T.; Ogawa, Y.; Imamaki, H.; Kusakabe, T. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009, 75, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Nickolas, T.L.; Forster, C.S.; Sise, M.E.; Barasch, N.; Solá-Del Valle, D.; Viltard, M.; Buchen, C.; Kupferman, S.; Carnevali, M.L.; Bennett, M. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012, 82, 718–722. [Google Scholar] [CrossRef]
- Parikh, C.; Jani, A.; Mishra, J.; Ma, Q.; Kelly, C.; Barasch, J.; Edelstein, C.; Devarajan, P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am. J. Transplant. 2006, 6, 1639–1645. [Google Scholar] [CrossRef]





| AKI | Healthy Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 118 | n = 16 | P | ||||||||||
| Age, years, mean ± S.E. | 62.2 | ± | 16.6 | 54.9 | ± | 2.9 | 0.379 | |||||
| BMI, kg/m2, mean ± S.E. | 24.1 | ± | 5.2 | 23.4 | ± | 0.8 | 0.596 | |||||
| Male gender, n (%) | 80 | ( | 67.8 | ) | 2 | ( | 12.5 | ) | <0.001 | |||
| Weight, kg, mean ± S.E. | 63.3 | ± | 15.8 | 60 | ± | 3.2 | 0.493 | |||||
| Complications, n (%) | ||||||||||||
| Diabetes | 37 | ( | 31.4 | ) | 1 | ( | 6.3 | ) | 0.054 | |||
| Hypertension | 78 | ( | 66.1 | ) | 7 | ( | 43.8 | ) | 0.294 | |||
| Dyslipidemia | 43 | ( | 36.4 | ) | 1 | ( | 6.3 | ) | 0.037 | |||
| Old myocardial infarction | 11 | ( | 9.3 | ) | 0 | ( | 0.0 | ) | 0.226 | |||
| Old cerebral infarction | 6 | ( | 5.1 | ) | 0 | ( | 0.0 | ) | 0.401 | |||
| Angina pectoris | 12 | ( | 10.2 | ) | 0 | ( | 0.0 | ) | 0.253 | |||
| Chronic obstructive pulmonary disease | 5 | ( | 4.2 | ) | 0 | ( | 0.0 | ) | 0.401 | |||
| Liver disease | 4 | ( | 3.4 | ) | 0 | ( | 0.0 | ) | 0.455 | |||
| Hematological data, mean ± S.E. | ||||||||||||
| Na, mEq/L | 137 | ± | 5.5 | 142 | ± | 0.5 | 0.005 | |||||
| K, mEq/L | 4.4 | ± | 0.9 | 4.2 | ± | 0.1 | 0.379 | |||||
| Cl, mEq/L | 103 | ± | 6.3 | 106 | ± | 0.6 | 0.124 | |||||
| BUN, mg/dL | 55 | ± | 33.2 | 14 | ± | 0.8 | <0.001 | |||||
| Creatinine, mg/dL | 4.52 | ± | 3.55 | 0.68 | ± | 0.04 | <0.001 | |||||
| eGFR, mL/min | 19.1 | ± | 16.7 | 79.9 | ± | 3.6 | <0.001 | |||||
| Hemoglobin, g/dL | 10.6 | ± | 2.9 | 13.3 | ± | 0.2 | 0.015 | |||||
| Platelets, x104/μL | 74.8 | ± | 109.3 | 26.1 | ± | 1.7 | 0.195 | |||||
| WBC, x103/μL | 6.9 | ± | 6.8 | 5.7 | ± | 0.4 | 0.462 | |||||
| CRP, mg/dL | 7.93 | ± | 9.0 | 0.24 | ± | 0.1 | 0.015 | |||||
| Urinalysis, mean ± S.E. | ||||||||||||
| Urinary protein, mg/dL | 245.1 | ± | 494.6 | - | - | |||||||
| NAG, IU/L | 33.6 | ± | 39.1 | - | - | |||||||
| α1MG, mg/L | 55.4 | ± | 66.7 | - | - | |||||||
| β2MG, mg/L | 12.5 | ± | 24.2 | - | - | |||||||
| NGAL, ng/mL | 1206.7 | ± | 2750.9 | - | - | |||||||
| KIM-1, ng/mL | 3.8 | ± | 7.5 | - | - | |||||||
| L-FABP, ng/mL | 168.4 | ± | 480.5 | - | - | |||||||
| FENa, % | 5.0 | ± | 7.4 | - | - | |||||||
| FEUrea, % | 36.5 | ± | 22.6 | - | - | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagayama, I.; Takayanagi, K.; Nagata, D.; Hasegawa, H.; Maeshima, A. Potential Utility of Urinary Follistatin as a Non-Invasive Indicator of Acute Tubular Damage in Patients with Acute Kidney Injury. Cells 2024, 13, 525. https://doi.org/10.3390/cells13060525
Nagayama I, Takayanagi K, Nagata D, Hasegawa H, Maeshima A. Potential Utility of Urinary Follistatin as a Non-Invasive Indicator of Acute Tubular Damage in Patients with Acute Kidney Injury. Cells. 2024; 13(6):525. https://doi.org/10.3390/cells13060525
Chicago/Turabian StyleNagayama, Izumi, Kaori Takayanagi, Daisuke Nagata, Hajime Hasegawa, and Akito Maeshima. 2024. "Potential Utility of Urinary Follistatin as a Non-Invasive Indicator of Acute Tubular Damage in Patients with Acute Kidney Injury" Cells 13, no. 6: 525. https://doi.org/10.3390/cells13060525