Zinc Finger Proteins in the War on Gastric Cancer: Molecular Mechanism and Clinical Potential
Abstract
:1. Introduction
2. Zinc Finger Proteins (ZFPs)
3. Biological Functions of ZFPs in GC
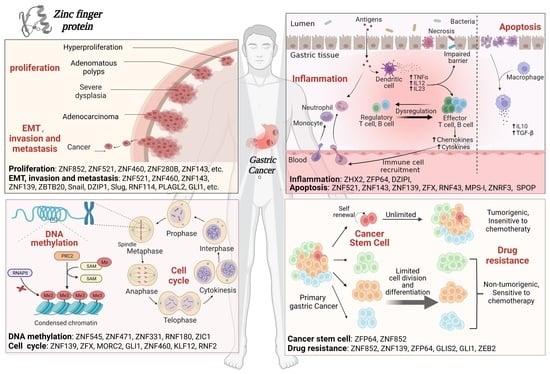
3.1. ZFPs Regulate Cell Proliferation
3.2. ZFPs Regulate EMT, Invasion and Metastasis
3.3. ZFPs Regulate Inflammation and Immune Infiltration
3.4. ZFPs Regulate Apoptosis
3.5. ZFPs Regulate the Cell Cycle
3.6. ZFPs Regulate DNA Methylation
3.7. ZFPs Regulate Cancer Stem Cells
3.8. ZFPs Regulate Drug Resistance
3.9. Other Pathway
4. MZF1: A Double-Edged Sword in GC
5. ZFPs in Prognosis Prediction and Diagnostic Means
6. Application of ZFPs in GC Therapeutic Intervention
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| ZFPs | Zinc finger proteins |
| EMT | Epithelial–mesenchymal transition |
| CSCs | Cancer stem cells |
| MZF1 | Myeloid zinc finger 1 |
| TFIIIA | Transcription factor IIIA |
| KRAB | Krüppel-associated box |
| BTB/POZ | Broad complex, tramtrack and bric-a-brac/poxvirus and zinc finger |
| TCF/LEF | T-cell factor/Lymphoid enhancer factor |
| ZFX | Zinc finger protein X-linked |
| DZIP1 | DAZ-interacting zinc finger protein 1 |
| RNF114 | E3 ubiquitin ligase ring finger protein 114 |
| PLAGL2 | Pleomorphic adenoma gene like-2 |
| EGFR | Epidermal growth factor receptor |
| APOC1 | Apolipoprotein C1 |
| KLF2 | Krüppel-like factor 2 |
| KLF4 | Krüppel-like factor 4 |
| KLF6 | Krüppel-like factor 6 |
| NKD2 | NKD inhibitor of WNT signaling pathway 2 |
| ZC3H15 | Zinc finger CCCH-type containing 15 |
| SPOP | Speckle-type POZ protein |
| MORC2 | Microrchidia family cysteine tryptophan (CW)-type zinc finger 2 |
| ZNRF3 | Zinc and ring finger 3 |
| ZBTB20 | Zinc finger and BTB domain-containing 20 |
| Slug | Zinc finger protein SNAI2 |
| GLI1 | GLI family zinc finger 1 |
| KLF8 | Krüppel-like factor 8 |
| CTCF | CCCTC-binding factor |
| ZEBs | E-box-binding proteins |
| KLF9 | Krüppel-like factor 9 |
| RNF180 | Ring finger protein 180 |
| TWIST1 | Twist family bHLH transcription factor 1 |
| ZHX2 | Zinc fingers and homeoboxes 2 |
| TNFAIP3 | TNFα-induced protein 3 |
| RNF43 | Ring finger protein 43 |
| MPS-1 | Metallopanstimulin-1 |
| KLF12 | Krüppel-like factor 12 |
| RNF2 | Ring finger protein 2 |
| CIP1 | Cyclin-dependent kinase-interacting protein 1 |
| ZIC1 | Zinc family member 1 |
| GLIS2 | GLIS family zinc finger 2 |
| Axl | AXL receptor tyrosine kinase |
| pTNM | Pathologic tumor–node–metastasis |
| ZBTB4 | Zinc finger and BTB domain-containing 4 |
References
- Rima, F.A.; Hussain, M.; Dewan, R.K.; Haque, M.N.; Sultana, T.; Chowdhury, F.; Aminullah, M.; Ferdous, J.N. Clinicopathologic Features of Gastric and Gastrooesophageal Junction Adenocarcinoma. Mymensingh Med. J. 2020, 29, 195–201. [Google Scholar] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Trujillo Rivera, A.; Sampieri, C.L.; Morales Romero, J.; Montero, H.; Acosta Mesa, H.G.; Cruz Ramírez, N.; Novoa Del Toro, E.M.; León Córdoba, K. Risk factors associated with gastric cancer in Mexico: Education, breakfast and chili. Rev. Esp. Enferm. Dig. 2018, 110, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Coburn, N.; Cosby, R.; Klein, L.; Knight, G.; Malthaner, R.; Mamazza, J.; Mercer, C.D.; Ringash, J. Staging and surgical approaches in gastric cancer: A systematic review. Cancer Treat. Rev. 2018, 63, 104–115. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Kuhara, A.; Sumi, A.; Chikasue, T.; Kawaguchi, A.; Tanoue, S.; Nagata, S.; Koganemaru, M.; Abe, T.; Kashihara, M.; Mitsuoka, M.; et al. Utility of non-contrast-enhanced magnetic resonance imaging in predicting preoperative clinical stage and prognosis in patients with thymic epithelial tumor. Jpn. J. Radiol. 2023, 41, 302–311. [Google Scholar] [CrossRef]
- Qiu, G.; Li, X.; Wei, C.; Che, X.; He, S.; Lu, J.; Wang, S.; Pang, K.; Fan, L. The Prognostic Role of SIRT1-Autophagy Axis in Gastric Cancer. Dis. Markers 2016, 2016, 6869415. [Google Scholar] [CrossRef]
- Stankovic, B.; Bjørhovde, H.A.K.; Skarshaug, R.; Aamodt, H.; Frafjord, A.; Müller, E.; Hammarström, C.; Beraki, K.; Bækkevold, E.S.; Woldbæk, P.R.; et al. Immune Cell Composition in Human Non-small Cell Lung Cancer. Front. Immunol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef]
- Machlab, D.; Burger, L.; Soneson, C.; Rijli, F.M.; Schübeler, D.; Stadler, M.B. monaLisa: An R/Bioconductor package for identifying regulatory motifs. Bioinformatics 2022, 38, 2624–2625. [Google Scholar] [CrossRef]
- An, G.; Feng, L.; Hou, L.; Li, X.; Bai, J.; He, L.; Gu, S.; Zhao, X. A bioinformatics analysis of zinc finger protein family reveals potential oncogenic biomarkers in breast cancer. Gene 2022, 828, 146471. [Google Scholar] [CrossRef] [PubMed]
- Jen, J.; Wang, Y.C. Zinc finger proteins in cancer progression. J. Biomed. Sci. 2016, 23, 53. [Google Scholar] [CrossRef]
- Karunasinghe, N. Zinc in Prostate Health and Disease: A Mini Review. Biomedicines 2022, 10, 3206. [Google Scholar] [CrossRef]
- Li, X.; Han, M.; Zhang, H.; Liu, F.; Pan, Y.; Zhu, J.; Liao, Z.; Chen, X.; Zhang, B. Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma. Biomark. Res. 2022, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sima, X.; Liu, X.; Chen, H. Zinc Finger Proteins: Functions and Mechanisms in Colon Cancer. Cancers 2022, 14, 5242. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.W. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals 2022, 35, 187–213. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Herman, J.G.; Linghu, E.; Yang, Y.; Fuks, F.; Zhou, F.; Song, L.; Guo, M. Methylation of ZNF331 is an independent prognostic marker of colorectal cancer and promotes colorectal cancer growth. Clin. Epigenet. 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Liu, D.; Song, H.; Li, Y.; Huang, R.; Liu, H.; Tang, K.; Jiao, N.; Liu, J. The Transcriptional Repressor PerR Senses Sulfane Sulfur by Cysteine Persulfidation at the Structural Zn(2+) Site in Synechococcus sp. PCC7002. Antioxidants 2023, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 Zinc Finger Proteins: Master Regulators of Abiotic Stress Responses in Plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef]
- Bu, S.; Lv, Y.; Liu, Y.; Qiao, S.; Wang, H. Zinc Finger Proteins in Neuro-Related Diseases Progression. Front. Neurosci. 2021, 15, 760567. [Google Scholar] [CrossRef]
- Li, C.; Xia, P.; Ma, Y.; Zhang, X.; Liu, Y. Expression pattern of ZNF33B in bovine ovaries and the effect of its polymorphism on superovulation traits. Arch. Anim. Breed. 2022, 65, 69–77. [Google Scholar] [CrossRef]
- Manohar, K.; Khandagale, P.; Patel, S.K.; Sahu, J.K.; Acharya, N. The ubiquitin-binding domain of DNA polymerase η directly binds to DNA clamp PCNA and regulates translesion DNA synthesis. J. Biol. Chem. 2022, 298, 101506. [Google Scholar] [CrossRef] [PubMed]
- Hillion, M.; Antelmann, H. Thiol-based redox switches in prokaryotes. Biol. Chem. 2015, 396, 415–444. [Google Scholar] [CrossRef]
- Cesaro, E.; Lupo, A.; Rapuano, R.; Pastore, A.; Grosso, M.; Costanzo, P. ZNF224 Protein: Multifaceted Functions Based on Its Molecular Partners. Molecules 2021, 26, 6296. [Google Scholar] [CrossRef] [PubMed]
- Bedard, J.E.; Haaning, A.M.; Ware, S.M. Identification of a novel ZIC3 isoform and mutation screening in patients with heterotaxy and congenital heart disease. PLoS ONE 2011, 6, e23755. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Cheray, M.; Keane, L.; Engskog-Vlachos, P.; Joseph, B. ULK3-dependent activation of GLI1 promotes DNMT3A expression upon autophagy induction. Autophagy 2022, 18, 2769–2780. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Majka, M. Interplay among SNAIL Transcription Factor, MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Regulation of Tumor Growth and Metastasis. Cancers 2020, 12, 209. [Google Scholar] [CrossRef]
- Ke, C.; Zhou, H.; Jiang, B.; Xie, X. Zinc finger protein 852 is essential for the proliferation, drug sensitivity, and self-renewal of gastric cancer cells. Cell Biol. Int. 2022, 46, 579–587. [Google Scholar] [CrossRef]
- Deng, J.; Liang, H.; Ying, G.; Dong, Q.; Zhang, R.; Yu, J.; Fan, D.; Hao, X. Poor survival is associated with the methylated degree of zinc-finger protein 545 (ZNF545) DNA promoter in gastric cancer. Oncotarget 2015, 6, 4482–4495. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, Y.; Du, W.; Lu, L.; Zhou, L.; Wang, H.; Kang, W.; Li, X.; Tao, Q.; Sung, J.J.; et al. Zinc-finger protein 545 is a novel tumour suppressor that acts by inhibiting ribosomal RNA transcription in gastric cancer. Gut 2013, 62, 833–841. [Google Scholar] [CrossRef]
- Huan, C.; Xiaoxu, C.; Xifang, R. Zinc Finger Protein 521, Negatively Regulated by MicroRNA-204-5p, Promotes Proliferation, Motility and Invasion of Gastric Cancer Cells. Technol. Cancer Res. Treat. 2019, 18, 1533033819874783. [Google Scholar] [CrossRef]
- Jin, X.S.; Ji, T.T.; Shi, Z.C.; Zhang, Q.Q.; Ye, F.P.; Yu, W.L.; Li, R.Z. Knockdown of ZNF479 inhibits proliferation and glycolysis of gastric cancer cells through regulating β-catenin/c-Myc signaling pathway. Kaohsiung J. Med. Sci. 2021, 37, 759–767. [Google Scholar] [CrossRef]
- Cao, L.; Wang, S.; Zhang, Y.; Wong, K.C.; Nakatsu, G.; Wang, X.; Wong, S.; Ji, J.; Yu, J. Zinc-finger protein 471 suppresses gastric cancer through transcriptionally repressing downstream oncogenic PLS3 and TFAP2A. Oncogene 2018, 37, 3601–3616. [Google Scholar] [CrossRef]
- An, L.; Liu, Y. ZNF460 mediates epithelial-mesenchymal transition to promote gastric cancer progression by transactivating APOC1 expression. Exp. Cell Res. 2023, 422, 113452. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liang, Q.Y.; Wang, J.; Cheng, Y.; Wang, S.; Poon, T.C.; Go, M.Y.; Tao, Q.; Chang, Z.; Sung, J.J. Zinc-finger protein 331, a novel putative tumor suppressor, suppresses growth and invasiveness of gastric cancer. Oncogene 2013, 32, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Yang, Z.; Cai, X.; Yao, G.; An, Y.; Wang, W.; Fan, Y.; Zeng, C.; Liu, K. ZNF280B promotes the growth of gastric cancer in vitro and in vivo. Oncol. Lett. 2018, 15, 5819–5824. [Google Scholar] [CrossRef]
- Wei, S.; Wang, L.; Zhang, L.; Li, B.; Li, Z.; Zhang, Q.; Wang, J.; Chen, L.; Sun, G.; Li, Q.; et al. ZNF143 enhances metastasis of gastric cancer by promoting the process of EMT through PI3K/AKT signaling pathway. Tumor Biol. 2016, 37, 12813–12821. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wei, S.; Sun, J.; Zhang, X.; He, L.; Zhang, L.; Xu, Z.; Chen, D. ZNF143 Suppresses Cell Apoptosis and Promotes Proliferation in Gastric Cancer via ROS/p53 Axis. Dis. Markers 2020, 2020, 5863178. [Google Scholar] [CrossRef]
- Fan, L.; Tan, B.; Li, Y.; Zhao, Q.; Liu, Y.; Wang, D.; Zhang, Z. Silencing of ZNF139-siRNA induces apoptosis in human gastric cancer cell line BGC823. Int. J. Clin. Exp. Pathol. 2015, 8, 12428–12436. [Google Scholar] [PubMed]
- Hao, Y.J.; Li, Y.; Fan, L.Q.; Zhao, Q.; Tan, B.B.; Jiao, Z.K.; Zhao, X.F.; Zhang, Z.D.; Wang, D. Role of RNA-interference-induced zinc finger protein 139 suppression in gastric cancer cell sensitivity to chemotherapeutic agents. Oncol. Lett. 2015, 10, 1333–1338. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.B.; Zhao, Q.; Fan, L.Q.; Wang, D.; Liu, Y. ZNF139 promotes tumor metastasis by increasing migration and invasion in human gastric cancer cells. Neoplasma 2014, 61, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Q.; Fan, L.Q.; Wang, L.L.; Tan, B.B.; Leng, Y.L.; Liu, Y.; Wang, D. Zinc finger protein 139 expression in gastric cancer and its clinical significance. World J. Gastroenterol. 2014, 20, 18346–18353. [Google Scholar] [CrossRef]
- Tan, B.; Li, Y.; Zhao, Q.; Fan, L.; Wang, D. ZNF139 increases multidrug resistance in gastric cancer cells by inhibiting miR-185. Biosci. Rep. 2018, 38, BSR20181023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, X.; Zhang, Z.; Zhang, X.; Chang, J.; Wu, Z.; Tang, W.; Gan, L.; Sun, M.; Li, J. MicroRNA-940 promotes tumor cell invasion and metastasis by downregulating ZNF24 in gastric cancer. Oncotarget 2015, 6, 25418–25428. [Google Scholar] [CrossRef]
- Cui, N.; Liu, J.; Xia, H.; Xu, D. LncRNA SNHG20 contributes to cell proliferation and invasion by upregulating ZFX expression sponging miR-495-3p in gastric cancer. J. Cell. Biochem. 2019, 120, 3114–3123. [Google Scholar] [CrossRef]
- Wu, S.; Lao, X.Y.; Sun, T.T.; Ren, L.L.; Kong, X.; Wang, J.L.; Wang, Y.C.; Du, W.; Yu, Y.N.; Weng, Y.R.; et al. Knockdown of ZFX inhibits gastric cancer cell growth in vitro and in vivo via downregulating the ERK-MAPK pathway. Cancer Lett. 2013, 337, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Guo, X.; Dai, X.; Wang, Z. Upregulation of ZHX2 predicts poor prognosis and is correlated with immune infiltration in gastric cancer. FEBS Open Bio 2021, 11, 1785–1798. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, P.; Yu, S.; Tang, C.; Wang, Y.; Shen, Z.; Chen, W.; Liu, T.; Cui, Y. Targeting ZFP64/GAL-1 axis promotes therapeutic effect of nab-paclitaxel and reverses immunosuppressive microenvironment in gastric cancer. J. Exp. Clin. Cancer Res. 2022, 41, 14. [Google Scholar] [CrossRef]
- Hou, J.; Huang, P.; Lan, C.; Geng, S.; Xu, M.; Liu, Y.; Chang, H.; Wang, Z.; Gu, H.; Wang, Y.; et al. ZC3H15 promotes gastric cancer progression by targeting the FBXW7/c-Myc pathway. Cell Death Discov. 2022, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Zhang, M.; Cheng, L.; Zhang, Y.; Wang, X. ZBTB20 promotes cell migration and invasion of gastric cancer by inhibiting IκBα to induce NF-κB activation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3862–3872. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Jin, X.; Liao, C.; Qiao, L.; Zhao, W. MicroRNA-301b-3p accelerates the growth of gastric cancer cells by targeting zinc finger and BTB domain containing 4. Pathol. Res. Pract. 2019, 215, 152667. [Google Scholar] [CrossRef]
- Taniuchi, T.; Mortensen, E.R.; Ferguson, A.; Greenson, J.; Merchant, J.L. Overexpression of ZBP-89, a zinc finger DNA binding protein, in gastric cancer. Biochem. Biophys. Res. Commun. 1997, 233, 154–160. [Google Scholar] [CrossRef]
- Romero, S.; Musleh, M.; Bustamante, M.; Stambuk, J.; Pisano, R.; Lanzarini, E.; Chiong, H.; Rojas, J.; Castro, V.G.; Jara, L.; et al. Polymorphisms in TWIST1 and ZEB1 Are Associated with Prognosis of Gastric Cancer Patients. Anticancer Res. 2018, 38, 3871–3877. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, Y.; Lu, Q.; Chen, J.; Zhang, J.; Liu, T.; Lv, N.; Luo, S. SPOP suppresses tumorigenesis by regulating Hedgehog/Gli2 signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2014, 33, 75. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Y.; Pan, C.; Yan, L.; Wang, Z.W.; Zhu, X. The emerging role of SPOP protein in tumorigenesis and cancer therapy. Mol. Cancer 2020, 19, 2. [Google Scholar] [CrossRef]
- Alves, C.C.; Rosivatz, E.; Schott, C.; Hollweck, R.; Becker, I.; Sarbia, M.; Carneiro, F.; Becker, K.F. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J. Pathol. 2007, 211, 507–515. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, N.; Zhou, Z.; Chen, J.; Han, S.; Zhang, X.; Bao, H.; Yuan, W.; Shu, X. PLAGL2 promotes the proliferation and migration of gastric cancer cells via USP37-mediated deubiquitination of Snail1. Theranostics 2021, 11, 700–714. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, J.; Shi, Q.; Liu, W.; Luo, B. EBV-miR-BART12 inhibits cell migration and proliferation by targeting Snail expression in EBV-associated gastric cancer. Arch. Virol. 2021, 166, 1313–1323. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, J.; Qiao, L.; Zhao, W. Deubiquitinase USP13 promotes the epithelial-mesenchymal transition and metastasis in gastric cancer by maintaining Snail protein. Pathol. Res. Pract. 2022, 229, 153705. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Wang, Y.; Li, J.; Liang, S.; Zhang, W.; Ma, Z.; Liu, S.; Zou, X. DZIP1 expressed in fibroblasts and tumor cells may affect immunosuppression and metastatic potential in gastric cancer. Int. Immunopharmacol. 2023, 117, 109886. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Zeng, Q.; Zhang, Y.; Tu, Y.; Chen, W.; Shu, X.; Wu, A.; Xiong, J.; Cao, Y.; et al. RNF114 Silencing Inhibits the Proliferation and Metastasis of Gastric Cancer. J. Cancer 2022, 13, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Ruan, B.; Chen, W.; Zheng, J.; Xu, B.; Jiang, P.; Miao, Z.; Li, F.; Guo, J.Y.; et al. MORC2 regulates C/EBPα-mediated cell differentiation via sumoylation. Cell Death Differ. 2019, 26, 1905–1917. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Shuai, L.; Zha, L.; He, M.; Huang, Z.; Wang, Z. Krüppel-like factor 4 negatively regulates β-catenin expression and inhibits the proliferation, invasion and metastasis of gastric cancer. Int. J. Oncol. 2012, 40, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Peng, M.; Li, Y.; Zhu, R.; Li, X.; Qian, Z. LINC00703 Acts as a Tumor Suppressor via Regulating miR-181a/KLF6 Axis in Gastric Cancer. J. Gastric Cancer 2019, 19, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Sangodkar, J.; Shi, J.; DiFeo, A.; Schwartz, R.; Bromberg, R.; Choudhri, A.; McClinch, K.; Hatami, R.; Scheer, E.; Kremer-Tal, S.; et al. Functional role of the KLF6 tumour suppressor gene in gastric cancer. Eur. J. Cancer 2009, 45, 666–676. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Zhang, H.; Xiang, Y.; Dai, Z.; Zhang, H.; Li, J.; Li, H.; Liao, X. LncRNA TPTEP1 inhibits the migration and invasion of gastric cancer cells through miR-548d-3p/KLF9/PER1 axis. Pathol. Res. Pract. 2022, 237, 154054. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.; Jiang, M.; Li, S.; Zhang, J.; Xu, Z.; Guo, D.; Gu, T.; Wang, B.; Xiao, L.; et al. KLF9 suppresses gastric cancer cell invasion and metastasis through transcriptional inhibition of MMP28. Faseb J. 2019, 33, 7915–7928. [Google Scholar] [CrossRef]
- Sun, H.; Gu, J.; Li, Z.; Liu, Q.; Lin, J.; Tian, Y.; Cao, J.; Qin, H.; Tang, Z. Low Expression of GLIS2 Gene Might Associate with Radiosensitivity of Gastric Cancer. J. Oncol. 2019, 2019, 2934925. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Tan, L.; Yin, Z.; Tao, K.; Wang, G.; Shi, W.; Gao, J. GLIS2 redundancy causes chemoresistance and poor prognosis of gastric cancer based on co-expression network analysis. Oncol. Rep. 2019, 41, 191–201. [Google Scholar] [CrossRef]
- Shan, Y.S.; Hsu, H.P.; Lai, M.D.; Hung, Y.H.; Wang, C.Y.; Yen, M.C.; Chen, Y.L. Cyclin D1 overexpression correlates with poor tumor differentiation and prognosis in gastric cancer. Oncol. Lett. 2017, 14, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, X.C.; Liu, T.; Li, W.J.; Xiang, J.G.; Xiao, D.; Zhang, Y.L.; Zheng, M.H.; Zhai, C.; Chen, L.; et al. GLI-1 facilitates the EMT induced by TGF-β1 in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6809–6815. [Google Scholar] [CrossRef]
- Shao, X.; Kuai, X.; Pang, Z.; Zhang, L.; Wu, L.; Xu, L.; Zhou, C. Correlation of Gli1 and HER2 expression in gastric cancer: Identification of novel target. Sci. Rep. 2018, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jia, W.; An, Q.; Cao, X.; Xiao, G. Bioinformatic Analysis of GLI1 and Related Signaling Pathways in Chemosensitivity of Gastric Cancer. Med. Sci. Monit. 2018, 24, 1847–1855. [Google Scholar] [CrossRef]
- Liu, C.; Deng, L.; Lin, J.; Zhang, J.; Huang, S.; Zhao, J.; Jin, P.; Xu, P.; Ni, P.; Xu, D.; et al. Zinc Finger Protein CTCF Regulates Extracellular Matrix (ECM)-Related Gene Expression Associated With the Wnt Signaling Pathway in Gastric Cancer. Front. Oncol. 2020, 10, 625633. [Google Scholar] [CrossRef]
- Du, B.; Liu, M.; Li, C.; Geng, X.; Zhang, X.; Ning, D.; Liu, M. The potential role of TNFAIP3 in malignant transformation of gastric carcinoma. Pathol. Res. Pract. 2019, 215, 152471. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Y.; Qu, X.; Che, X.; Li, C.; Fan, Y.; Wan, X.; Ma, R.; Hou, K.; Zhou, H.; et al. miR-200a enhances TRAIL-induced apoptosis in gastric cancer cells by targeting A20. Cell Biol. Int. 2018, 42, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Wang, Y.; Zhao, G.; Xie, R.; Liu, C.; Xiao, X.; Wu, K.; Nie, Y.; Zhang, H.; et al. KLF8 involves in TGF-beta-induced EMT and promotes invasion and migration in gastric cancer cells. J. Cancer Res. Clin. Oncol. 2013, 139, 1033–1042. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Sun, W.; Du, Y.; He, W.; Guo, S.; Chen, L.; Zhao, Z.; Wang, P.; Liang, H.; et al. RNF180 mediates STAT3 activity by regulating the expression of RhoC via the proteasomal pathway in gastric cancer cells. Cell Death Dis. 2020, 11, 881. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhang, S.; Li, M. Plasma Methylated RNF180 for Noninvasive Diagnosis of Gastric Cancer. Biomed Res. Int. 2022, 2022, 6548945. [Google Scholar] [CrossRef]
- Sun, W.; Ma, G.; Zhang, L.; Wang, P.; Zhang, N.; Wu, Z.; Dong, Y.; Cai, F.; Chen, L.; Liu, H.; et al. DNMT3A-mediated silence in ADAMTS9 expression is restored by RNF180 to inhibit viability and motility in gastric cancer cells. Cell Death Dis. 2021, 12, 428. [Google Scholar] [CrossRef]
- Xu, J.; Song, J.; Wang, T.; Zhu, W.; Zuo, L.; Wu, J.; Guo, J.; Yang, X. A combination of methylation and protein markers is capable of detecting gastric cancer detection by combined markers. Epigenomics 2021, 13, 1557–1570. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.S.; Lee, H.S.; Hong, S.; Rajasekaran, N.; Wang, L.H.; Choi, J.S.; Shin, Y.K. Upregulation of SMAD4 by MZF1 inhibits migration of human gastric cancer cells. Int. J. Oncol. 2017, 50, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, X.; Pan, Y.; Tian, R.; Lin, B.; Jiang, G.; Chen, K.; He, Y.; Zhang, L.; Zhai, W.; et al. Transcription Factor Myeloid Zinc-Finger 1 Suppresses Human Gastric Carcinogenesis by Interacting with Metallothionein 2A. Clin. Cancer Res. 2019, 25, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, J.; Li, Y.; Xu, Y. MZF1 Transcriptionally Activated MicroRNA-328-3p Suppresses the Malignancy of Stomach Adenocarcinoma via Inhibiting CD44. J. Immunol. Res. 2022, 2022, 5819295. [Google Scholar] [CrossRef]
- Mudduluru, G.; Vajkoczy, P.; Allgayer, H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol. Cancer Res. 2010, 8, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jiao, W.; Mei, H.; Song, H.; Li, D.; Xiang, X.; Chen, Y.; Yang, F.; Li, H.; Huang, K.; et al. miRNA-337-3p inhibits gastric cancer progression through repressing myeloid zinc finger 1-facilitated expression of matrix metalloproteinase 14. Oncotarget 2016, 7, 40314–40328. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.; Barsuhn, S.; Behrens, H.M.; Krüger, S.; Röcken, C. The tumor biological significance of RNF43 and LRP1B in gastric cancer is complex and context-dependent. Sci. Rep. 2023, 13, 3191. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Qin, H.Z.; Xi, H.Q.; Wei, B.; Xia, S.Y.; Chen, L. RNF43 Inhibits Cancer Cell Proliferation and Could be a Potential Prognostic Factor for Human Gastric Carcinoma. Cell. Physiol. Biochem. 2015, 36, 1835–1846. [Google Scholar] [CrossRef]
- Sohn, S.H.; Sul, H.J.; Kim, B.; Kim, H.S.; Kim, B.J.; Lim, H.; Kang, H.S.; Soh, J.S.; Kim, K.C.; Cho, J.W.; et al. RNF43 and PWWP2B inhibit cancer cell proliferation and are predictive or prognostic biomarker for FDA-approved drugs in patients with advanced gastric cancer. J. Cancer 2021, 12, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, A.; Xi, H.; Li, J.; Xu, W.; Zhang, Y.; Zhang, K.; Cui, J.; Wu, X.; Wei, B.; et al. Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-β/catenin signaling pathway. Stem Cell Res. Ther. 2017, 8, 98. [Google Scholar] [CrossRef]
- Radaszkiewicz, T.; Bryja, V. Protease associated domain of RNF43 is not necessary for the suppression of Wnt/β-catenin signaling in human cells. Cell Commun. Signal. 2020, 18, 91. [Google Scholar] [CrossRef]
- Neumeyer, V.; Brutau-Abia, A.; Allgäuer, M.; Pfarr, N.; Weichert, W.; Falkeis-Veits, C.; Kremmer, E.; Vieth, M.; Gerhard, M.; Mejías-Luque, R. Loss of RNF43 Function Contributes to Gastric Carcinogenesis by Impairing DNA Damage Response. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1071–1094. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Kang, Y.T.; Chen, C.; Xu, F.F.; Wang, H.N.; Jin, R. Combination of TNM staging and pathway based risk score models in patients with gastric cancer. J. Cell. Biochem. 2018, 119, 3608–3617. [Google Scholar] [CrossRef]
- Lakshmi Ch, N.P.; Sivagnanam, A.; Raja, S.; Mahalingam, S. Molecular basis for RASSF10/NPM/RNF2 feedback cascade-mediated regulation of gastric cancer cell proliferation. J. Biol. Chem. 2021, 297, 100935. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Han, Y.; Yao, R.; Yue, L.; Xu, Y.; Zhang, J. Rnf2 knockdown reduces cell viability and promotes cell cycle arrest in gastric cancer cells. Oncol. Lett. 2017, 13, 3817–3822. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Li, N.; Wu, Y.; Zhang, J.; Xu, R.; Ming, H. LBX2-AS1 up-regulated by NFIC boosts cell proliferation, migration and invasion in gastric cancer through targeting miR-491-5p/ZNF703. Cancer Cell Int. 2020, 20, 136. [Google Scholar] [CrossRef]
- Du, Y.; Chen, Y.; Wang, F.; Gu, L. miR-137 plays tumor suppressor roles in gastric cancer cell lines by targeting KLF12 and MYO1C. Tumor Biol. 2016, 37, 13557–13569. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhang, A.; Yang, Y.; Wu, D. Circ-RNF111 aggravates the malignancy of gastric cancer through miR-876-3p-dependent regulation of KLF12. World J. Surg. Oncol. 2021, 19, 259. [Google Scholar] [CrossRef]
- Cao, D.; Luo, Y.; Qin, S.; Yu, M.; Mu, Y.; Ye, G.; Yang, N.; Cong, Z.; Chen, J.; Qin, J.; et al. Metallopanstimulin-1 (MPS-1) mediates the promotion effect of leptin on colorectal cancer through activation of JNK/c-Jun signaling pathway. Cell Death Dis. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Qu, Y.; Zhang, Q.; Wei, M.; Liu, C.X.; Chen, X.H.; Yan, M.; Zhu, Z.G.; Liu, B.Y.; Chen, G.Q.; et al. Knockdown of metallopanstimulin-1 inhibits NF-κB signaling at different levels: The role of apoptosis induction of gastric cancer cells. Int. J. Cancer 2012, 130, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Qu, Y.; Li, J.F.; Chen, X.H.; Liu, B.Y.; Gu, Q.L.; Zhu, Z.G. In vitro and in vivo evidence of metallopanstimulin-1 in gastric cancer progression and tumorigenicity. Clin. Cancer Res. 2006, 12, 4965–4973. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Q.; Zhou, X. LAMA4 expression is activated by zinc finger E-box-binding homeobox 1 and independently predicts poor overall survival in gastric cancer. Oncol. Rep. 2018, 40, 1725–1733. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Jiang, H.; Li, Y.; Lu, C.; Chen, T.; Wang, Y.; Wang, X.; Sun, W.; Pu, Z.; et al. The aryl sulfonamide indisulam inhibits gastric cancer cell migration by promoting the ubiquitination and degradation of the transcription factor ZEB1. J. Biol. Chem. 2023, 299, 103025. [Google Scholar] [CrossRef]
- Xu, C.; Cui, H.; Li, H.; Wu, Y.; An, H.; Guo, C. Long non-coding RNA ZEB2-AS1 expression is associated with disease progression and predicts outcome in gastric cancer patients. J. BUON 2019, 24, 663–671. [Google Scholar]
- Geng, D.M.; Kan, X.M.; Zhang, W.W. Effect of ZEB2 silencing on cisplatin resistance in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1746–1752. [Google Scholar]
- Pei, L.; He, X.; Li, S.; Sun, R.; Xiang, Q.; Ren, G.; Xiang, T. KRAB zinc-finger protein 382 regulates epithelial-mesenchymal transition and functions as a tumor suppressor, but is silenced by CpG methylation in gastric cancer. Int. J. Oncol. 2018, 53, 961–972. [Google Scholar] [CrossRef]
- Qin, H.; Cai, A.; Xi, H.; Yuan, J.; Chen, L. ZnRF3 induces apoptosis of gastric cancer cells by antagonizing Wnt and Hedgehog signaling. Panminerva Med. 2015, 57, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lan, J.; Wang, W.; Shi, Q.; Lan, Y.; Cheng, Z.; Guan, H. ZNRF3 acts as a tumour suppressor by the Wnt signalling pathway in human gastric adenocarcinoma. J. Mol. Histol. 2013, 44, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Z.; Xue, M.; Si, J.; Chen, S. Zic1 Promoter Hypermethylation in Plasma DNA Is a Potential Biomarker for Gastric Cancer and Intraepithelial Neoplasia. PLoS ONE 2015, 10, e0133906. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, S.; Xue, M.; Du, Q.; Cai, J.; Jin, H.; Si, J.; Wang, L. ZIC1 modulates cell-cycle distributions and cell migration through regulation of sonic hedgehog, PI(3)K and MAPK signaling pathways in gastric cancer. BMC Cancer 2012, 12, 290. [Google Scholar] [CrossRef]
- Feng, R.; Li, Z.; Wang, X.; Ge, G.; Jia, Y.; Wu, D.; Ji, Y.; Wang, C. Silenced lncRNA SNHG14 restrains the biological behaviors of bladder cancer cells via regulating microRNA-211-3p/ESM1 axis. Cancer Cell Int. 2021, 21, 67. [Google Scholar] [CrossRef]
- Khan, A.A.; Qahtani, S.A.; Dawasaz, A.A.; Saquib, S.A.; Asif, S.M.; Ishfaq, M.; Kota, M.Z.; Ibrahim, M. Management of an extensive odontogenic keratocyst: A rare case report with 10-year follow-up. Medicine 2019, 98, e17987. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Desiderio, D.M. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med. Genom. 2010, 3, 13. [Google Scholar] [CrossRef]
- Hou, J.; Xu, M.; Gu, H.; Pei, D.; Liu, Y.; Huang, P.; Chang, H.; Cui, H. ZC3H15 promotes glioblastoma progression through regulating EGFR stability. Cell Death Dis. 2022, 13, 55. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Q.; Li, R.; Luo, J.; Yuan, D.; Song, J.; Sun, Y.; Long, T.; Yang, Z. SPOP Regulates The Biological Mechanism Of Ovarian Cancer Cells Through The Hh Signaling Pathway. OncoTargets Ther. 2019, 12, 9239–9248. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Roubille, S.; Lomonte, P.; Schaeffer, L. Microrchidia CW-Type Zinc Finger 2, a Chromatin Modifier in a Spectrum of Peripheral Neuropathies. Front. Cell. Neurosci. 2022, 16, 896854. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Niu, L.; Xu, L.; Guo, Y.; Wang, L.; Guo, C. Suppression of G6PD induces the expression and bisecting GlcNAc-branched N-glycosylation of E-Cadherin to block epithelial-mesenchymal transition and lymphatic metastasis. Br. J. Cancer 2020, 123, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Korona-Głowniak, I.; Forma, A.; Maani, A.; Sitarz, E.; Rahnama-Hezavah, M.; Radzikowska, E.; Portincasa, P. Mechanisms of the Epithelial-Mesenchymal Transition and Tumor Microenvironment in Helicobacter pylori-Induced Gastric Cancer. Cells 2020, 9, 1055. [Google Scholar] [CrossRef]
- Bure, I.V.; Nemtsova, M.V.; Zaletaev, D.V. Roles of E-cadherin and Noncoding RNAs in the Epithelial-mesenchymal Transition and Progression in Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 2870. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, H.; Chen, B.; Xu, W.; Zhao, J.; Huang, C.; Xing, Y.; Lv, H.; Nie, C.; Wang, J.; et al. Overview on the Role of E-Cadherin in Gastric Cancer: Dysregulation and Clinical Implications. Front. Mol. Biosci. 2021, 8, 689139. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, I.; Cantelli, G.; Bruce, F.; Sanz-Moreno, V. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research 2016, 5, 783. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Zeng, Y.; Xie, Y.; Zhou, J. Gastrin/CCK-B Receptor Signaling Promotes Cell Invasion and Metastasis by Upregulating MMP-2 and VEGF Expression in Gastric Cancer. J. Cancer 2022, 13, 134–145. [Google Scholar] [CrossRef]
- Fang, X.; Bai, Y.; Zhang, L.; Ding, S. Silencing circSLAMF6 represses cell glycolysis, migration, and invasion by regulating the miR-204-5p/MYH9 axis in gastric cancer under hypoxia. Biosci. Rep. 2020, 40, BSR20201275. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, W.; Qiu, J.; Chen, F. lncRNA SNHG4 promotes cell proliferation, migration, invasion and the epithelial-mesenchymal transition process via sponging miR-204-5p in gastric cancer. Mol. Med. Rep. 2021, 23, 85. [Google Scholar] [CrossRef]
- Shen, X.; Hu, X.; Mao, J.; Wu, Y.; Liu, H.; Shen, J.; Yu, J.; Chen, W. The long noncoding RNA TUG1 is required for TGF-β/TWIST1/EMT-mediated metastasis in colorectal cancer cells. Cell Death Dis. 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Zavros, Y.; Merchant, J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, M.; Zhang, M.; Wang, Y.; Zhang, Y.; Wang, S.; Zhang, N. Effects of Inflammation on the Immune Microenvironment in Gastric Cancer. Front. Oncol. 2021, 11, 690298. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Khalaf, A.A.; Tohamy, A.F.; Mohammed, E.R.; Farroh, K.Y. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int. J. Nanomed. 2019, 14, 4723–4739. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, S.; Wang, J. miR-217 inhibits the migration and invasion of HeLa cells through modulating MAPK1. Int. J. Mol. Med. 2019, 44, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, Q. A multi-omics-based investigation of the immunological and prognostic impact of necroptosis-related genes in patients with hepatocellular carcinoma. J. Clin. Lab. Anal. 2022, 36, e24346. [Google Scholar] [CrossRef]
- Tang, C.T.; Lin, X.L.; Wu, S.; Liang, Q.; Yang, L.; Gao, Y.J.; Ge, Z.Z. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell. Signal. 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hou, Y.C.; Huang, J.; Fang, J.Y.; Xiong, H. Itraconazole induces apoptosis and cell cycle arrest via inhibiting Hedgehog signaling in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 50. [Google Scholar] [CrossRef]
- Li, J.; Cheng, C.; Xu, J.; Zhang, T.; Tokat, B.; Dolios, G.; Ramakrishnan, A.; Shen, L.; Wang, R.; Xu, P.X. The transcriptional coactivator Eya1 exerts transcriptional repressive activity by interacting with REST corepressors and REST-binding sequences to maintain nephron progenitor identity. Nucleic Acids Res. 2022, 50, 10343–10359. [Google Scholar] [CrossRef]
- Park, E.J.; Min, K.J.; Choi, K.S.; Kubatka, P.; Kruzliak, P.; Kim, D.E.; Kwon, T.K. Chloroquine enhances TRAIL-mediated apoptosis through up-regulation of DR5 by stabilization of mRNA and protein in cancer cells. Sci. Rep. 2016, 6, 22921. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhou, S.L.; Li, J.K.; Chen, P.N.; Zhao, X.K.; Wang, L.D.; Li, X.L.; Zhou, F.Y. Identification of a seven-cell cycle signature predicting overall survival for gastric cancer. Aging 2022, 14, 3989–3999. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, T.J.M.; Kleinjans, J.C.S.; Jennen, D.G.J. From multi-omics integration towards novel genomic interaction networks to identify key cancer cell line characteristics. Sci. Rep. 2021, 11, 10542. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Li, Y.; Wang, R.; Li, Y.; Zhang, H.; Ren, D.; Liu, H.; Kang, C.; Chen, J. HOTAIR, a long noncoding RNA, is a marker of abnormal cell cycle regulation in lung cancer. Cancer Sci. 2018, 109, 2717–2733. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, L.; He, L.; Wei, Q.; Bi, J.; Wang, Y.; Yu, L.; He, M.; Zhao, L.; Wei, M. Identification of a novel cell cycle-related gene signature predicting survival in patients with gastric cancer. J. Cell. Physiol. 2019, 234, 6350–6360. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Tateishi, K.; Kanai, F.; Watabe, H.; Kondo, S.; Guleng, B.; Tanaka, Y.; Asaoka, Y.; Jazag, A.; Imamura, J.; et al. p53-Independent negative regulation of p21/cyclin-dependent kinase-interacting protein 1 by the sonic hedgehog-glioma-associated oncogene 1 pathway in gastric carcinoma cells. Cancer Res. 2005, 65, 10822–10829. [Google Scholar] [CrossRef]
- Pan, X.; Li, C.; Cai, Y.; Wu, S. Comprehensive Pan-Cancer Analysis Reveals the Role of UHRF1-Mediated DNA Methylation and Immune Infiltration in Renal Cell Carcinoma. J. Oncol. 2022, 2022, 3842547. [Google Scholar] [CrossRef]
- Lin, S.; Yi, S.; Qiu, P. Comprehensive analysis of TCGA data reveals correlation between DNA methylation and alternative splicing. BMC Genom. 2022, 23, 758. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Perez, R.; Fernández-Olavarria, A.; Diaz-Sanchez, R.M.; Gutierrez-Perez, J.L.; Serrera-Figallo, M.; Torres-Lagares, D. Stem cells and oral surgery: A systematic review. J. Clin. Exp. Dent. 2019, 11, e1181–e1189. [Google Scholar] [CrossRef]
- El Baba, R.; Herbein, G. Immune Landscape of CMV Infection in Cancer Patients: From "Canonical" Diseases Toward Virus-Elicited Oncomodulation. Front. Immunol. 2021, 12, 730765. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yue, W.; Wei, B.; Wang, N.; Li, T.; Guan, L.; Shi, S.; Zeng, Q.; Pei, X.; Chen, L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS ONE 2011, 6, e17687. [Google Scholar] [CrossRef]
- Qi, W.; Yang, Z.; Feng, Y.; Li, H.; Che, N.; Liu, L.; Xuan, Y. Gli1 regulates stemness characteristics in gastric adenocarcinoma. Diagn. Pathol. 2020, 15, 60. [Google Scholar] [CrossRef]
- Yu, D.; Shin, H.S.; Lee, Y.S.; Lee, D.; Kim, S.; Lee, Y.C. Genistein attenuates cancer stem cell characteristics in gastric cancer through the downregulation of Gli1. Oncol. Rep. 2014, 31, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, B.; Wang, B.; Wu, D.; Wang, C.; Gao, Y.; Liang, W.; Xi, H.; Wang, X.; Chen, L. MiR-144-3p inhibits gastric cancer progression and stemness via directly targeting GLI2 involved in hedgehog pathway. J. Transl. Med. 2021, 19, 432. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, J.F.; Huang, F.K.; Zhang, L.; He, Q.L.; Qian, H.Y.; Lai, H.L. GLI2 induces PDGFRB expression and modulates cancer stem cell properties of gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3857–3865. [Google Scholar] [PubMed]
- Qiu, X.; Wang, Q.; Song, H.; Shao, D.; Xue, J. circ_103809 promotes breast cancer progression by regulating the PI3K/AKT signaling pathway. Oncol. Lett. 2020, 19, 3725–3730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Cao, M.; Mei, S.; Guo, S.; Zhang, W.; Ji, N.; Zhao, Z. Trends in metabolic signaling pathways of tumor drug resistance: A scientometric analysis. Front. Oncol. 2022, 12, 981406. [Google Scholar] [CrossRef]
- Lampropoulou, D.I.; Pliakou, E.; Aravantinos, G.; Filippou, D.; Gazouli, M. The Role of Exosomal Non-Coding RNAs in Colorectal Cancer Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1473. [Google Scholar] [CrossRef]
- Yu, B.; Gu, D.; Zhang, X.; Liu, B.; Xie, J. The role of GLI2-ABCG2 signaling axis for 5Fu resistance in gastric cancer. J. Genet. Genom. 2017, 44, 375–383. [Google Scholar] [CrossRef]
- Hsieh, A.L.; Walton, Z.E.; Altman, B.J.; Stine, Z.E.; Dang, C.V. MYC and metabolism on the path to cancer. Semin. Cell Dev. Biol. 2015, 43, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef]
- Gupta, A.; Ajith, A.; Singh, S.; Panday, R.K.; Samaiya, A.; Shukla, S. PAK2-c-Myc-PKM2 axis plays an essential role in head and neck oncogenesis via regulating Warburg effect. Cell Death Dis. 2018, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Gao, P.; Liu, Y.C.; Semenza, G.L.; Dang, C.V. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell. Biol. 2007, 27, 7381–7393. [Google Scholar] [CrossRef]
- Toller, I.M.; Neelsen, K.J.; Steger, M.; Hartung, M.L.; Hottiger, M.O.; Stucki, M.; Kalali, B.; Gerhard, M.; Sartori, A.A.; Lopes, M.; et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14944–14949. [Google Scholar] [CrossRef]
- Hromas, R.; Collins, S.J.; Hickstein, D.; Raskind, W.; Deaven, L.L.; O’Hara, P.; Hagen, F.S.; Kaushansky, K. A retinoic acid-responsive human zinc finger gene, MZF-1, preferentially expressed in myeloid cells. J. Biol. Chem. 1991, 266, 14183–14187. [Google Scholar] [CrossRef]
- Wu, C.W.; Li, A.F.; Chi, C.W.; Lai, C.H.; Huang, C.L.; Lo, S.S.; Lui, W.Y.; Lin, W.C. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002, 22, 1071–1078. [Google Scholar] [PubMed]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Kuo, M.C.; Kuo, P.C.; Mi, Z. Myeloid zinc finger-1 regulates expression of cancer-associated fibroblast and cancer stemness profiles in breast cancer. Surgery 2019, 166, 515–523. [Google Scholar] [CrossRef]
- Wu, L.; Han, L.; Zhou, C.; Wei, W.; Chen, X.; Yi, H.; Wu, X.; Bai, X.; Guo, S.; Yu, Y.; et al. TGF-β1-induced CK17 enhances cancer stem cell-like properties rather than EMT in promoting cervical cancer metastasis via the ERK1/2-MZF1 signaling pathway. FEBS J. 2017, 284, 3000–3017. [Google Scholar] [CrossRef]
- Inoue, M.; Takahashi, K.; Niide, O.; Shibata, M.; Fukuzawa, M.; Ra, C. LDOC1, a novel MZF-1-interacting protein, induces apoptosis. FEBS Lett. 2005, 579, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Kim, Y.H. Identifying molecular drivers of gastric cancer through next-generation sequencing. Cancer Lett. 2013, 340, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.E.; Wang, P.L.; Yin, S.C.; Zhang, C.; Hou, W.B.; Xu, H.M. Thirty-year trends in clinicopathologic characteristics and prognosis after gastrectomy for gastric cancer: A single institution in Northern China. J. Cancer 2020, 11, 1056–1062. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Li, D.; Wang, J.; Wang, G. Prognostic significance of microsatellite instability-associated pathways and genes in gastric cancer. Int. J. Mol. Med. 2018, 42, 149–160. [Google Scholar] [CrossRef]
- Tamura, T.; Ohira, M.; Tanaka, H.; Muguruma, K.; Toyokawa, T.; Kubo, N.; Sakurai, K.; Amano, R.; Kimura, K.; Shibutani, M.; et al. Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res. 2015, 35, 5369–5376. [Google Scholar]
- Wu, J.; Liu, X.; Cai, H.; Wang, Y. Prediction of tumor recurrence after curative resection in gastric carcinoma based on bcl-2 expression. World J. Surg. Oncol. 2014, 12, 40. [Google Scholar] [CrossRef]
- Yuza, K.; Nagahashi, M.; Watanabe, S.; Takabe, K.; Wakai, T. Hypermutation and microsatellite instability in gastrointestinal cancers. Oncotarget 2017, 8, 112103–112115. [Google Scholar] [CrossRef]
- Cho, S.J.; Kook, M.C.; Lee, J.H.; Shin, J.Y.; Park, J.; Bae, Y.K.; Choi, I.J.; Ryu, K.W.; Kim, Y.W. Peroxisome proliferator-activated receptor γ upregulates galectin-9 and predicts prognosis in intestinal-type gastric cancer. Int. J. Cancer 2015, 136, 810–820. [Google Scholar] [CrossRef]
- He, Q.; Li, G.; Wang, X.; Wang, S.; Hu, J.; Yang, L.; He, Y.; Pan, Y.; Yu, D.; Wu, Y. A Decrease of Histone Deacetylase 6 Expression Caused by Helicobacter Pylori Infection is Associated with Oncogenic Transformation in Gastric Cancer. Cell. Physiol. Biochem. 2017, 42, 1326–1335. [Google Scholar] [CrossRef]
- Szczepanik, A.; Sierzega, M.; Drabik, G.; Pituch-Noworolska, A.; Kołodziejczyk, P.; Zembala, M. CD44(+) cytokeratin-positive tumor cells in blood and bone marrow are associated with poor prognosis of patients with gastric cancer. Gastric Cancer 2019, 22, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Märkl, B.; Grosser, B.; Bauer, K.; Vlasenko, D.; Schenkirsch, G.; Probst, A.; Kriening, B. Ultrastaging Using Ex Vivo Sentinel Lymph Node Mapping and One-Step Nucleic Acid Amplification (OSNA) in Gastric Cancer: Experiences of a European Center. Cancers 2021, 13, 2683. [Google Scholar] [CrossRef]
- Zheng, X.; Song, X.; Shao, Y.; Xu, B.; Chen, L.; Zhou, Q.; Hu, W.; Zhang, D.; Wu, C.; Tao, M.; et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A meta-analysis. Oncotarget 2017, 8, 57386–57398. [Google Scholar] [CrossRef]
- Yi, J.; Ren, L.; Wu, J.; Li, W.; Zheng, X.; Du, G.; Wang, J. Apolipoprotein C1 (APOC1) as a novel diagnostic and prognostic biomarker for gastric cancer. Ann. Transl. Med. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.L.; Crabtree, J.E.; Yoshimura, T.; Clayton, C.L.; Dixon, M.F.; Robinson, P.A. Increased zinc finger protein zFOC1 transcripts in gastric cancer compared with normal gastric tissue. Mol. Pathol. 2003, 56, 167–171. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.K.; Qin, S.; Yamaguchi, K.; Kim, I.H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef]
- Herbst, R.S.; Arkenau, H.T.; Santana-Davila, R.; Calvo, E.; Paz-Ares, L.; Cassier, P.A.; Bendell, J.; Penel, N.; Krebs, M.G.; Martin-Liberal, J.; et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019, 20, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Liu, L.; Liu, S.; Liang, L.; Chen, Y.; Zhu, Z. Circular RNA circ_0006282 Contributes to the Progression of Gastric Cancer by Sponging miR-155 to Upregulate the Expression of FBXO22. OncoTargets Ther. 2020, 13, 1001–1010. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, X.; Du, Y.; Tang, M.; Guo, X.; Chen, Z.; Liu, Y.; Lu, Y. Insulin-Like Growth Factor 2 mRNA-Binding Protein 3 and Its Related Molecules as Potential Biomarkers in Small-Cell Lung Cancer. Biomed Res. Int. 2022, 2022, 5774339. [Google Scholar] [CrossRef]
- Nie, H.; Mu, J.; Wang, J.; Li, Y. miR-195-5p regulates multi-drug resistance of gastric cancer cells via targeting ZNF139. Oncol. Rep. 2018, 40, 1370–1378. [Google Scholar] [CrossRef]
- Qiang, F.; Li, J. CircCSNK1G1 Contributes to the Tumorigenesis of Gastric Cancer by Sponging miR-758 and Regulating ZNF217 Expression. Cancer Manag. Res. 2021, 13, 5027–5038. [Google Scholar] [CrossRef] [PubMed]
- Inoue, J.; Inazawa, J. Cancer-associated miRNAs and their therapeutic potential. J. Hum. Genet. 2021, 66, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Chen, Z.; Chen, W.; Yuan, L.; Liu, B. The prognostic value of circular RNA regulatory genes in competitive endogenous RNA network in gastric cancer. Cancer Gene Ther. 2021, 28, 1175–1187. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Kamali, M.J.; Abak, A.; Shoorei, H.; Taheri, M. LncRNA ZFAS1: Role in tumorigenesis and other diseases. Biomed. Pharmacother. 2021, 142, 111999. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; He, L.; Li, Y.; Tan, Y.; Zhang, F.; Xu, H. Silencing of lncRNA ZFAS1 inhibits malignancies by blocking Wnt/β-catenin signaling in gastric cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 456–465. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Xu, W.; He, L.; Tan, Y.; Xu, H. Long non-coding RNA ZFAS1 regulates the malignant progression of gastric cancer via the microRNA-200b-3p/Wnt1 axis. Biosci. Biotechnol. Biochem. 2019, 83, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Yu, X.; Huang, M.; Wang, Y.; Xie, M.; Ma, H.; Wang, Z.; De, W.; Sun, M. Long noncoding RNA ZFAS1 promotes gastric cancer cells proliferation by epigenetically repressing KLF2 and NKD2 expression. Oncotarget 2017, 8, 38227–38238. [Google Scholar] [CrossRef]
- Jiang, T.; Mei, L.; Yang, X.; Sun, T.; Wang, Z.; Ji, Y. Biomarkers of gastric cancer: Current advancement. Heliyon 2022, 8, e10899. [Google Scholar] [CrossRef]
- Pan, L.; Liang, W.; Fu, M.; Huang, Z.H.; Li, X.; Zhang, W.; Zhang, P.; Qian, H.; Jiang, P.C.; Xu, W.R.; et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 2017, 143, 991–1004. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, F.; Chen, H.; Tan, Q.; Qiu, S.; Chen, S.; Jing, W.; Yu, M.; Liang, C.; Ye, S.; et al. Increased expression of long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal transition of gastric cancer. Aging 2016, 8, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, P.; Hei, Y.; Li, S.; Wang, J.; Lv, X.; Zhang, J. Long non-coding RNA-ZNF281 promotes cancer cell migration and invasion in gastric cancer via downregulation of microRNA-124. Oncol. Lett. 2020, 19, 1849–1855. [Google Scholar] [CrossRef]
- Yan, S.M.; Tang, J.J.; Huang, C.Y.; Xi, S.Y.; Huang, M.Y.; Liang, J.Z.; Jiang, Y.X.; Li, Y.H.; Zhou, Z.W.; Ernberg, I.; et al. Reduced expression of ZDHHC2 is associated with lymph node metastasis and poor prognosis in gastric adenocarcinoma. PLoS ONE 2013, 8, e56366. [Google Scholar] [CrossRef]
- Han, Y.; Duan, B.; Wu, J.; Zheng, Y.; Gu, Y.; Cai, X.; Lu, C.; Wu, X.; Li, Y.; Gu, X. Analysis of Time Series Gene Expression and DNA Methylation Reveals the Molecular Features of Myocardial Infarction Progression. Front. Cardiovasc. Med. 2022, 9, 912454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Bai, C.; Nie, Y.; Lin, G. Multi-Modality Treatment for Patients With Metastatic Gastric Cancer: A Real-World Study in China. Front. Oncol. 2019, 9, 1155. [Google Scholar] [CrossRef]
- Fulton, S.L.; Maze, I. Translational Molecular Approaches in Substance Abuse Research. Handb. Exp. Pharmacol. 2020, 258, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, C.; Liu, Y. Establishing a cancer driver gene signature-based risk model for predicting the prognoses of gastric cancer patients. Aging 2022, 14, 2383–2399. [Google Scholar] [CrossRef]
- Shi, C.; Yu, Y.; Yan, J.; Hu, C. The added value of radiomics from dual-energy spectral CT derived iodine-based material decomposition images in predicting histological grade of gastric cancer. BMC Med. Imaging 2022, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.; Li, F.; Wang, Y.; Wei, M. AFF3 is a novel prognostic biomarker and a potential target for immunotherapy in gastric cancer. J. Clin. Lab. Anal. 2022, 36, e24437. [Google Scholar] [CrossRef]



| ZFPs | Aliases | Expression | Main Roles | Targets | Ref |
|---|---|---|---|---|---|
| ZNF852 | - | ↑ | Cell proliferation Drug resistance Cancer stem cells | EGFR | [33] |
| ZNF545 | KIAA1948, MGC45380, ZFP82 | ↓ | Cell proliferation DNA methylation | rRNA, Heterochromatin protein 1β, Trimethylated histone H3 | [34,35] |
| ZNF521 | EHZF, Evi3 | ↑ | Cell proliferation EMT, invasion and metastasis | MicroRNA-106-5p | [36] |
| ZNF479 | HKr19 | ↑ | Cell proliferation Glycolysis | β-catenin/c-Myc pathway | [37] |
| ZNF471 | KIAA1396, Z1971, Zfp78 | ↓ | Cell proliferation EMT, invasion and metastasis DNA methylation | KAP1, TFAP2A, PLS3 | [38] |
| ZNF460 | IKZF2, Helios, ZNFN1A2 | ↑ | Cell proliferation EMT, invasion and metastasis Cell cycle | APOC1 | [39] |
| ZNF331 | RITA, ZNF361, ZNF463 | ↓ | Cell proliferation EMT, invasion and metastasis DNA methylation | DSTN, EIF5A, GARS, DDX5, STAM, UQCRFS1, SET, DSTN, ACTR3, SSBP1, PNPT1 | [21,40] |
| ZNF280B | 5′OY11.1, SUHW2, ZNF279, ZNF632 | ↑ | Cell proliferation | --- | [41] |
| ZNF143 | pHZ-1, SBF, STAF | ↑ | Cell proliferation Apoptosis EMT, invasion and metastasis | ROS/p53 axis, PI3K/Akt pathway | [42,43] |
| ZNF139 | ZNF36, ZSCAN33, ZKSCAN1, KOX18, PHZ-37 | ↑ | Cell proliferation EMT, invasion and metastasis Drug resistance Apoptosis Cell cycle | Bcl-2, Bax, Caspase-3, MDR1/P-gp, MRP-2, Bcl-185, ANXA2, Fascin, PDXK, MMP-2, MMP-9, ICAM-1, TIMP-1 | [44,45,46,47,48] |
| ZNF24 | KOX17, ZFP191, ZNF191, ZSCAN3 | ↓ | EMT, invasion and metastasis | MicroRNA-940 | [29,49] |
| ZFX | ZNF926 | ↑ | Cell proliferation Apoptosis Cell cycle | ERK-MAPK pathway, SNHG20/miR-495-3p/ZFX axis, FTX/miR-144/ZFX axis | [50,51] |
| ZHX2 | KIAA0854 | ↑ | Inflammation | [52] | |
| ZFP64 | ZNF338, dJ548G19.1dJ831D17.1FLJ10734 FLJ12628 | ↑ | EMT, invasion and metastasis Drug resistance Cancer stem cells Immunosuppress | GAL-1 | [53] |
| ZC3H15 | LEREPO4 | ↑ | Cell proliferation EMT, invasion and metastasis | FBXW7, c-Myc, FBXW7/c-Myc pathway | [54] |
| ZBTB20 | DKFZp566F123, DPZF, ODA-8S, ZNF288 | ↑ | Cell proliferation EMT, invasion and metastasis | NF-κBp65, MMP-2, MMP-9, IκBα | [55] |
| ZBTB4 | KAISO-L1, KIAA1538, ZNF903 | ↓ | Cell proliferation | miR-301b-3p | [56] |
| ZBP89 | BERF-1, BFCOL1, HT-BETA, pHZ-52, ZFP148, ZNF148 | ↑ | Cell proliferation | gERE, Sp1, EGF | [57] |
| TWIST1 | ACS3, bHLHa38, BPES2, BPES3, CRS, CRS1, H-twist, SCS, TWIST | EMT, invasion and metastasis | E-cadherin, Snail, Zeb and Twist | [58] | |
| SPOP | BTBD32, TEF2 | ↓ | Cell proliferation EMT, invasion and metastasis Apoptosis | Hh/GLI2 pathway | [59,60] |
| Snail | SNAI1, SNAIL1, SLUGH2, SNA, SNAH | ↑ | EMT, invasion and metastasis | USP13, EBV-miR-BART12, E-cadherin, TNF-α, NF-κB pathway | [61,62,63,64] |
| DZIP1 | DZIP, KIAA0996 | ↑ | Cell proliferation EMT, invasion and metastasis Immunosuppress | CAFs | [65] |
| Slug | SNAI2, SNAIL2, SLUH1, SLUGH, | ↑ | EMT, invasion and metastasis | E-cadherin, SIP1, SIP2, Snail | [61] |
| RNF114 | PSORS12, ZNF313 | ↑ | Cell proliferation EMT, invasion and metastasis | EGR1, miR-218-5p, EGR1 | [66] |
| PLAGL2 | ZNF900 | ↑ | Cell proliferation EMT, invasion and metastasis | USP37, Snail | [62] |
| MORC2 | AC004542.C22.1, KIAA0852, ZCW3, ZCWCC1 | ↑ | Cell proliferation EMT, invasion and metastasis Cell cycle | C/EBPα | [67] |
| KLF4 | EZF, GKLF | ↓ | Cell proliferation EMT, invasion and metastasis | β-catenin, E-cadherin, MMP2 | [68] |
| KLF6 | BCD1, COPEB, CPBP, GBFPAC1, ST12, Zf9 | ↓ | Cell proliferation Cell cycle | p21, c-Myc, LINC00703, miR-181a/KLF6 axis | [69,70] |
| KLF9 | BTEB1 | ↓ | EMT, invasion and metastasis | MMP28, TPTEP1/KLF9/PER1 axis, miR-548d-3p | [71,72] |
| GLIS2 | NPHP7 | ↑ | Drug resistance Cell cycle | Cyclin D1, 𝛽-catenin, TCF/LEF | [73,74,75] |
| GLI1 | GLI | ↑ | EMT, invasion and metastasis Drug resistance Apoptosis Cancer stem cell | E-cadherin, Vimentin, TGF-β1, GANT, PI3K/Akt/mTOR pathway, PD-L1, Akt-mTOR-p1S70K, HER2, SMO | [76,77,78] |
| GLI2 | - | ↑ | Cell cycle Cancer stem cell Drug resistance | GANT61, Hh/Gli pathway, CyclinD1, p21, Sp1, hTERT/Sp1/Gli1 axis | [59] |
| CTCF | CFAP108, FAP108 | ↑ | Cell proliferation EMT, invasion and metastasis | Wnt pathway, COL1A1, COLA31 | [79] |
| TNFAIP3 | A20, OTUD7C | ↓ | Apoptosis Inflammation | NF-κB pathway, TNF-α, IL-1, IL-1, IL-6, IL-8, CARMA1-Bcl-10-MALT1 pathway, TNFR1, TNFR2 | [80,81] |
| KLF8 | ↑ | EMT, invasion and metastasis | E-cadherin, Vimentin, TGF-β1 | [82] | |
| RNF180 | - | ↓ | DNA methylation EMT, invasion and metastasis | ZIC2 | [83,84,85,86] |
| MZF1 | MZF-1, MZF1B, Zfp98, ZNF42, ZSCAN6 | ↓ | Apoptosis EMT, invasion and metastasis | MT2A, NF-κB pathway, LODC1, SMAD4, miRNA-337-3p, MMP-14 | [87,88,89,90,91] |
| RNF43 | DKFZp781H0392, FLJ20315, URCC | ↓ | Apoptosis EMT, invasion and metastasis Cancer stem cell | Wnt signaling pathway | [13,92,93,94,95,96,97,98] |
| RNF2 | BAP-1, BAP1, DING, HIPI3, RING1B, RING2 | ↑ | Cell cycle Cell proliferation | RASSF10/NPM/RNF2 feedback | [99,100] |
| ZNF703 | FLJ14299, NLZ1, ZEPPO1, ZNF503L, Zpo1 | ↑ | Cell proliferation | LBX2-AS1, NFIC, miR-491-5p | [101] |
| KLF12 | AP-2rep, AP2REP, HSPC122 | ↑ | Cell proliferation Cell cycle | AP-2-alpha gene, A32, miR-137 | [102,103] |
| MPS-1 | RPS27, S27 | ↑ | EMT, invasion and metastasis Apoptosis | MPS-1/NF-κB/Gadd45β pathway, JNK, Caspase3 | [104,105,106] |
| ZEB1 | AREB6, BZP, FECD6, NIL-2-A, PPCD3, TCF8, ZEB, Zfhep, Zfhx1a | ↑ | EMT, invasion and metastasis | LAMA4, MMP2, indisulam, RBM39, DCAF15, Yes, miR-200a, miR200b, miR-200c, miR-141, miR-429, N-cadherin, BZLF1 | [58,80,107,108] |
| ZEB2 | KIAA0569, SIP-1, SIP1, ZFHX1B | ↑ | Cell proliferation EMT, invasion and metastasis Drug resistance | MMP-2, MMP-9 | [109,110] |
| ZNF382 | FLJ14686, KS1 | ↓ | EMT, invasion and metastasis DNA methylation Cancer stem cell | SNAIL, Vimentin, Twist, NOTCH1, NOTCH2, NOTCH3, NOTCH4, HES-1, JAG1, MMP2, MMP11, NANOG, OCT4, SOX2, E-cadherin | [111] |
| ZNRF3 | BK747E2.3, FLJ22057, KIAA1133, RNF203 | ↓ | Apoptosis Cell proliferation | β-catenin, TCF-4, Wnt/β-catenin/TCF pathway | [112,113] |
| ZIC1 | ZIC, ZNF201 | ↓ | EMT, invasion and metastasis DNA methylation Cell cycle | Shh signaling, PI3K/Akt signaling, MAPK/ERK signaling | [114,115] |
| miRs | Expression | Targets | Functions | Ref |
|---|---|---|---|---|
| miR-204-5p | ↓ | ZNF521 | Negatively regulates ZNF521 Cell proliferation EMT, invasion and metastasis Apoptosis | [36] |
| miR-195-5p | ↓ | ZNF139 | Negatively regulates ZNF139 Cell proliferation Drug resistance | [191] |
| miR-940 | ↑ | ZNF24 | Negatively regulates ZNF24 Cell proliferation EMT, invasion and metastasis | [49] |
| miR-301b-3p | ↑ | ZBTB4 | Negatively regulates ZBTB4 Cell proliferation Apoptosis Cell cycle | [56] |
| EBV-miR-BARTs | ↓ | Snail | Negatively regulates Snail Cell proliferation EMT, invasion and metastasis Apoptosis Cell cycle | [63] |
| miR-181a | ↑ | KLF6 | Negatively regulates KLF6 Cell proliferation EMT, invasion and metastasis | [69] |
| miR-337-3p | ↓ | MZF1 | Negatively regulates MZF1 Cell proliferation EMT, invasion and metastasis | [91] |
| miR-758 | ↓ | ZNF217 | circCSNK1G1 negatively regulates miR-758, while miR-758 negatively regulates ZNF217 Cell proliferation | [192] |
| miR-495-3p | ↓ | ZFX | lncRNA SNHG20 negatively regulates miR-495-3p, while miR-495-3p negatively regulates ZFX Cell proliferation (SNHG20/miR-495-3p/ZFX axis) EMT, invasion and metastasis | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, X.; Lin, X.; Chen, H. Zinc Finger Proteins in the War on Gastric Cancer: Molecular Mechanism and Clinical Potential. Cells 2023, 12, 1314. https://doi.org/10.3390/cells12091314
Liu S, Liu X, Lin X, Chen H. Zinc Finger Proteins in the War on Gastric Cancer: Molecular Mechanism and Clinical Potential. Cells. 2023; 12(9):1314. https://doi.org/10.3390/cells12091314
Chicago/Turabian StyleLiu, Shujie, Xingzhu Liu, Xin Lin, and Hongping Chen. 2023. "Zinc Finger Proteins in the War on Gastric Cancer: Molecular Mechanism and Clinical Potential" Cells 12, no. 9: 1314. https://doi.org/10.3390/cells12091314