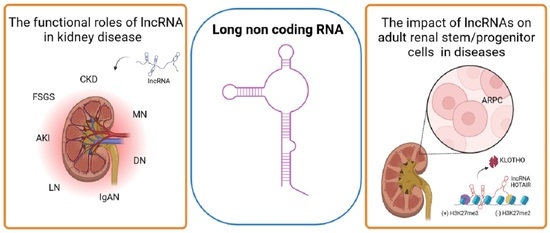
The Mission of Long Non-Coding RNAs in Human Adult Renal Stem/Progenitor Cells and Renal Diseases
Abstract
:1. Introduction
2. Biogenesis, Structure, and Functions of Long Non-Coding RNAs (LncRNAs)
3. The Role of LncRNA in Kidney Disease
3.1. Diabetic Nephropathy
3.2. Acute Kidney Injury
3.3. Chronic Kidney Disease
3.4. Glomerulonephritis
| lncRNA | Tissue/Cells | Disease | Mechanism |
|---|---|---|---|
| ARAP1-AS1 and ARAP1-AS2 | HK-2 | DN | Both enhance the mRNA expression of ARAP1, a member of the renin-angiotensin system [62,63] |
| ENST-00000453774.1 | HK-2 | CKD | Reduces ECM-bound proteins fibronectin and collagen I [99] |
| Erbb4 –Immunoreactivity | TECs and elongated, fibroblast-like cells | CKD | Regulates the expression of collagen I, α–smooth muscle actin, and Smad7 [89] |
| GAS5 | Kidney cells | CKD | Has anti-pyroptotic properties [100] |
| H19 | ECs, TECs | AKI due to renal I/R injury | Upregulates miR-30a-5p [81] |
| HK-2 | CKD | Affects TNF-α and IL-6 expression [93,95] | |
| HOTAIR | PBMCs | IgAN | Possible involvement in the NGF signaling pathway and Toll-like receptor pathways [104] |
| HOXA-AS2 | HK-2 | AKI | Hinders the Wnt/β-catenin and NF-κB pathways [75] |
| ICR | Renal Proximal Tubular Cells | IgAN | Is involved in the Akt/mTOR signaling pathway [108] |
| LINC00667 | Renal Tubular Epithelial Cell | CKD | Regulates apoptosis, cell proliferation, autophagy, and EMT [97] |
| lncRNA 9884 | HK-2 | AKI induced by nephrotoxic agents | Promotes the production of inflammatory cytokines via NF-κB [79] |
| lncRN A6406 | PTEC | AKI | Modulates miR-687/PTEN signaling [76,77] |
| lncRNA G21551 | Exosomes | IgAN | Regulates the expression of FCGR3B [105] |
| lncRNA PTTG3P | Peripheral B cells | IgAN | Promotes B-cell growth, IL-1β, and IL-8 production by regulating miR-383 [106] |
| LOC105375913 | Renal tubular cells | FSGS | Increases the level of snail and tubulointerstitial fibrosis [101] |
| LOC105374325 | Podocytes | FSGS | Increases the level of Bax and Bak genes and causes cell apoptosis [102] |
| MALAT1 | HK-2 | AKI | Activates NF-κB [70] |
| ECs, TECs | AKI due to renal I/R injury | Negatively regulates the expression of IL-6, TNF-α, and NF-kB [82] | |
| Kidney cells | CKD | Promotes pyroptosis by downregulating miR-23c [100] | |
| NEAT1 | Mesangial cells | DN | Activates Akt/mTOR signaling, and represses TGF-β1, FN, and COL-IV expression [64] |
| ECs, TECs | AKI due to renal I/R injury | Downregulates miR-27a-3p [83] | |
| Renal Tubular Epithelial Cell | MN | Inhibits Noxa-mediated anti-apoptotic activity [112] | |
| PRINS | Renal tubular cells | AKI due to renal I/R injury | Regulates the production of RANTES [84] |
| PVT1 | Mesangial cells | DN | Increased expression of extracellular matrix proteins [57,58] |
| HK-2 | CKD | Is involved in the TGF-β signaling pathway [92] | |
| RP11-2B6.2 | Renal cells | LN | Intervenes in the IFN-I pathway through epigenetic inhibition of SOCS1 [113] |
| TCONS_00088786, TCONS_01496394 | Kidney tissue | Tubular ischemia and CKD | Affect the expression of genes related to renal fibrosis [87,88] |
| TUG1 | Podocytes | DN | Modulates mitochondrial bioenergetics [59] |
| HK-2 | AKI | Interacts with Nrf2 [60] | |
| XIST | ECs, TECs | AKI due to renal I/R7 injury | Induces apoptosis and inflammation [80] |
| HK-2 | Renal calcinosis | Influence the expression of some inflammatory factors [96] | |
| Kidney tissues and cultured podocytes | MN | Proapoptotic effect through upregulation of Toll-like receptor 4 and downregulation of miR-217 [109,110] | |
| XLOC-032768 | HK-2 | AKI induced by nephrotoxic agents | Regulates tumor necrosis factor TNF-α [78] |
4. The Impact of LncRNAs on Stem Cells in Disease
5. The LncRNAs in Human Adult Renal Stem/Progenitor Cells
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crick, F.H. On Protein Synthesis. Symp. Soc. Exp. Biol. 1958, 12, 138–163. [Google Scholar] [PubMed]
- Chen, L.-L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Mattick, J.S. The State of Long Non-Coding RNA Biology. Non-Coding RNA 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, Y.; Xu, Q.; Zhang, Y.; Bei, S.; Ding, Y.; Zhang, X.; Feng, L. Diagnostic value of circulating lncRNAs for gastric cancer: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1058028. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Firoozabadi, A.D.; Peymani, M.; Ghaedi, K. Circulating long noncoding RNAs as novel bio-tools: Focus on autoimmune diseases. Hum. Immunol. 2022, 83, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Luo, H.; Li, D.; Wang, S.; Xuan, L.; Sun, L. Research advances on circulating long noncoding RNAs as biomarkers of cardiovascular diseases. Int. J. Cardiol. 2022, 353, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tawfick, A.; Matboli, M.; Shamloul, S.; Agwa, S.H.A.; Saad, M.; Shaker, H.; Selim, M.M.Y.; Salim, M.S.; Radwan, A.; Shorbagy, A.A.; et al. Predictive urinary RNA biomarkers of kidney injury after extracorporeal shock wave lithotripsy. World J. Urol. 2022, 40, 1561–1567. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, J.; Wang, Z.; Cao, Z.-Y.; Wang, L. Serum LncRNA PANDAR may Act as a Novel Serum Biomarker of Diabetic Nephropathy in Patients with Type 2 Diabetes. Clin. Lab. 2020, 1, 66. [Google Scholar] [CrossRef]
- Flippot, R.; Beinse, G.; Boilève, A.; Vibert, J.; Malouf, G.G. Long non-coding RNAs in genitourinary malignancies: A whole new world. Nat. Rev. Urol. 2019, 16, 484–504. [Google Scholar] [CrossRef]
- Morán, I.; Akerman, I.; Van De Bunt, M.; Xie, R.; Benazra, M.; Nammo, T.; Arnes, L.; Nakić, N.; García-Hurtado, J.; Rodríguez-Seguí, S. Human β Cell Transcriptome Analysis Uncovers LncRNAs That Are Tissue-Specific, Dynamically Regulated, and Abnormally Expressed in Type 2 Diabetes. Cell Metab. 2012, 16, 435–448. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Chen, L.-L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef]
- Preker, P.; Nielsen, J.; Kammler, S.; Lykke-Andersen, S.; Christensen, M.S.; Mapendano, C.K.; Schierup, M.H.; Jensen, T.H. RNA Exosome Depletion Reveals Transcription Upstream of Active Human Promoters. Science 2008, 322, 1851–1854. [Google Scholar] [CrossRef] [Green Version]
- De Santa, F.; Barozzi, I.; Mietton, F.; Ghisletti, S.; Polletti, S.; Tusi, B.K.; Muller, H.; Ragoussis, J.; Wei, C.-L.; Natoli, G. A Large Fraction of Extragenic RNA Pol II Transcription Sites Overlap Enhancers. PLoS Biol. 2010, 8, e1000384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, M.T.Y.; Cho, H.; Lesch, H.P.; Gosselin, D.; Heinz, S.; Tanaka-Oishi, Y.; Benner, C.; Kaikkonen, M.U.; Kim, A.S.; Kosaka, M.; et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 2013, 498, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, C.A.; Drost, J.; Wijchers, P.J.; van de Werken, H.; de Wit, E.; Vrielink, J.A.O.; Elkon, R.; Melo, S.A.; Léveillé, N.; Kalluri, R.; et al. eRNAs Are Required for p53-Dependent Enhancer Activity and Gene Transcription. Mol. Cell 2012, 49, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Gunawardane, L.; Niazi, F.; Jahanbani, F.; Chen, X.; Valadkhan, S. A Novel RNA Motif Mediates the Strict Nuclear Localization of a Long Noncoding RNA. Mol. Cell. Biol. 2014, 34, 2318–2329. [Google Scholar] [CrossRef] [Green Version]
- Lubelsky, Y.; Ulitsky, I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature 2018, 555, 107–111. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Goff, L.; Trapnell, C.; Williams, A.; Henao-Mejia, J.; Sun, L.; McClanahan, P.; Hendrickson, D.G.; Sauvageau, M.; Kelley, D.R.; et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat. Struct. Mol. Biol. 2014, 21, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.-F.; Yin, Q.-F.; Chen, T.; Zhang, Y.; Zhang, X.-O.; Wu, Z.; Zhang, S.; Wang, H.-B.; Ge, J.; Lu, X.; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014, 24, 513–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Sun, B.K.; Erwin, J.A.; Song, J.-J.; Lee, J.T. Polycomb Proteins Targeted by a Short Repeat RNA to the Mouse X Chromosome. Science 2008, 322, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Pintacuda, G.; Masui, O.; Koseki, Y.; Gdula, M.; Cerase, A.; Brown, D.; Mould, A.; Innocent, C.; Nakayama, M. PCGF3/5–PRC1 Initiates Polycomb Recruitment in X Chromosome Inactivation. Science 2017, 356, 1081–1084. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, P.; Li, W.; Lin, C.-H.; Yang, J.; Shang, C.; Nurnberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.-Y.; Lin, C.-J.; et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boque-Sastre, R.; Soler, M.; Oliveira-Mateos, C.; Portela, A.; Moutinho, C.; Sayols, S.; Villanueva, A.; Esteller, M.; Guil, S. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc. Natl. Acad. Sci. USA 2015, 112, 5785–5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postepska-Igielska, A.; Giwojna, A.; Gasri-Plotnitsky, L.; Schmitt, N.; Dold, A.; Ginsberg, D.; Grummt, I. LncRNA Khps1 Regulates Expression of the Proto-oncogene SPHK1 via Triplex-Mediated Changes in Chromatin Structure. Mol. Cell 2015, 60, 626–636. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Q.; Luo, K.; Zhou, Q. The 7SK Small Nuclear RNA Inhibits the CDK9/Cyclin T1 Kinase to Control Transcription. Nature 2021, 414, 317–322. [Google Scholar] [CrossRef]
- Mariner, P.D.; Walters, R.D.; Espinoza, C.A.; Drullinger, L.F.; Wagner, S.D.; Kugel, J.F.; Goodrich, J.A. Human Alu RNA Is a Modular Transacting Repressor of mRNA Transcription during Heat Shock. Mol. Cell 2008, 29, 499–509. [Google Scholar] [CrossRef]
- Kim, Y.K.; Furic, L.; DesGroseillers, L.; Maquat, L.E. Mammalian Staufen1 Recruits Upf1 to Specific mRNA 3′UTRs so as to Elicit mRNA Decay. Cell 2005, 120, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Maquat, L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 2011, 470, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kopp, F.; Chang, T.-C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tichon, A.; Gil, N.; Lubelsky, Y.; Solomon, T.H.; Lemze, D.; Itzkovitz, S.; Stern-Ginossar, N.; Ulitsky, I. A conserved abundant cytoplasmic long noncoding RNA modulates repression by Pumilio proteins in human cells. Nat. Commun. 2016, 7, 12209. [Google Scholar] [CrossRef] [Green Version]
- Kleaveland, B.; Shi, C.Y.; Stefano, J.; Bartel, D.P. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 2018, 174, 350–362.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Jia, L.; Wen, X.; Du, Z.; Wang, C.; Hao, Y.; Yu, D.; Zhou, L.; Chen, N.; et al. Profiling the long noncoding RNA interaction network in the regulatory elements of target genes by chromatin in situ reverse transcription sequencing. Genome Res. 2019, 29, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, D.S.; Padam, K.S.R.; Chakrabarty, S.; An, N.K.; Radhakrishnan, R. The regulatory role of HOX interacting lncRNA in oral cancer—An in silico analysis. J. Oral Pathol. Med. 2022, 51, 684–693. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-Free Nucleic Acids as Biomarkers in Cancer Patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Lijun, Q.; Shao, Y.; Zhang, X.; Zheng, T.; Miao, M.; Qin, L.; Wang, B.; Ye, G.; Xiao, B.; Guo, J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol. 2015, 36, 2007–2012. [Google Scholar] [CrossRef]
- Weber, D.G.; Johnen, G.; Casjens, S.; Bryk, O.; Pesch, B.; Jöckel, K.-H.; Kollmeier, J.; Brüning, T. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res. Notes 2013, 6, 518–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Wang, F.; Shen, J.; Sun, Y.; Xu, W.; Lu, J.; Wei, M.; Xu, C.; Wu, C.; Zhang, Z.; et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur. J. Cancer 2013, 49, 2949–2959. [Google Scholar] [CrossRef]
- Xie, H.; Ma, H.; Zhou, D. Plasma HULC as a Promising Novel Biomarker for the Detection of Hepatocellular Carcinoma. Biomed. Res. Int. 2013, 2013, 136106. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Watanabe, Y.; Sato, Y.; Maehata, T.; Itoh, F. Non-Invasive Early Molecular Detection of Gastric Cancers. Cancers 2020, 12, 2880. [Google Scholar] [CrossRef]
- Elsayed, E.T.; Salem, P.E.; Darwish, A.M.; Fayed, H.M. Plasma long non-coding RNA HOTAIR as a potential biomarker for gastric cancer. Int. J. Biol. Markers 2018, 33, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Yang, Y.; Shao, Y.; Zhang, H. Guo Plasma LncRNA-GACAT2 Is a Valuable Marker for the Screening of Gastric Cancer. Oncol. Lett. 2016, 12, 4845–4849. [Google Scholar] [CrossRef] [Green Version]
- Svoboda, M.; Slyskova, J.; Schneiderová, M.; Makovicky, P.; Bielik, L.; Levy, M.; Lipska, L.; Hemmelova, B.; Kala, Z.; Protivankova, M.; et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 2014, 35, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Wang, D.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.-H.; Qi, P.; Du, X. Long non-coding RNAs in cancer invasion and metastasis. Mod. Pathol. 2015, 28, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, Y.; Segura, V.; Marín-Béjar, O.; Athie, A.; Marchese, F.P.; González, J.; Bujanda, L.; Guo, S.; Matheu, A.; Huarte, M. Genome-wide analysis of the human p53 transcriptional network unveils a lncRNA tumour suppressor signature. Nat. Commun. 2014, 5, 5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating MicroRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arun, G.; Diermeier, S.; Akerman, M.; Chang, K.C.; Wilkinson, J.E.; Hearn, S.; Kim, Y.; MacLeod, A.R.; Krainer, A.R.; Norton, L.; et al. Differentiation of Mammary Tumors and Reduction in Metastasis upon Malat1 LncRNA Loss. Genes Dev. 2016, 30, 34–51. [Google Scholar] [CrossRef] [Green Version]
- Bichu, P.; Nistala, R.; Khan, A.; Sowers, J.R.; Whaley-Connell, A. Angiotensin Receptor Blockers for the Reduction of Proteinuria in Diabetic Patients with Overt Nephropathy: Results from the AMADEO Study. Vasc. Health Risk Manag. 2009, 5, 129–140. [Google Scholar] [PubMed]
- Himmelfarb, J.; Tuttle, K.R. New Therapies for Diabetic Kidney Disease. N. Engl. J. Med. 2013, 369, 2549–2550. [Google Scholar] [CrossRef]
- Hanson, R.L.; Craig, D.W.; Millis, M.P.; Yeatts, K.A.; Kobes, S.; Pearson, J.V.; Lee, A.M.; Knowler, W.C.; Nelson, R.G.; Wolford, J.K. Identification of PVT1 as a Candidate Gene for End-Stage Renal Disease in Type 2 Diabetes Using a Pooling-Based Genome-Wide Single Nucleotide Polymorphism Association Study. Diabetes 2007, 56, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.L.; DiStefano, J.K. Functional Characterization of the Plasmacytoma Variant Translocation 1 Gene (PVT1) in Diabetic Nephropathy. PLoS ONE 2011, 6, e18671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.L.; DiStefano, J.K. The role of non-coding RNAs in diabetic nephropathy: Potential applications as biomarkers for disease development and progression. Diabetes Res. Clin. Pr. 2013, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Badal, S.S.; Ye, Z.; Wang, Y.; Ayanga, B.A.; Galvan, D.; Green, N.H.; Chang, B.H.; Overbeek, P.A.; Danesh, F.R. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J. Clin. Investig. 2016, 126, 4205–4218. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Susztak, K. The long noncoding RNA Tug1 connects metabolic changes with kidney disease in podocytes. J. Clin. Investig. 2016, 126, 4072–4075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lv, X.; Fan, Q.; Wang, X.; Xu, L.; Lu, X.; Chen, T. Analysis of circulating lncRNA expression profiles in patients with diabetes mellitus and diabetic nephropathy: Differential expression profile of circulating lncRNA. Clin. Nephrol. 2019, 92, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.; Wen, S.; Yang, Y.; Li, X.; Fan, Q. The Effect of LncRNA-ARAP1-AS2/ARAP1 on High Glucose-Induced Cytoskeleton Rearrangement and Epithelial-Mesenchymal Transition in Human Renal Tubular Epithelial Cells. J Cell Physiol. 2020, 235, 5787–5795. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, Y.; Ge, X.; Xu, B.; Peng, W.; Jiang, X.; Shen, L.; Xia, L. Long noncoding RNA NEAT1 accelerates the proliferation and fibrosis in diabetic nephropathy through activating Akt/mTOR signaling pathway. J. Cell. Physiol. 2018, 234, 11200–11207. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, L.; Zhang, F.; Gu, J.; Pan, G. LncRNA TapSAKI promotes inflammation injury in HK-2 cells and urine derived sepsis-induced kidney injury. J. Pharm. Pharmacol. 2019, 71, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Jiang, X.; Wang, H.; Pan, G. LncRNA HOX Transcript Antisense RNA Accelerated Kidney Injury Induced by Urine-Derived Sepsis through the MiR-22/High Mobility Group Box 1 Pathway. Life Sci. 2018, 210, 185–191. [Google Scholar] [CrossRef]
- Pang, X.; Feng, G.; Shang, W.; Liu, L.; Li, J.; Feng, Y.; Xie, H.; Wang, J. Inhibition of lncRNA MEG3 protects renal tubular from hypoxia-induced kidney injury in acute renal allografts by regulating miR-181b/TNF-α signaling pathway. J. Cell. Biochem. 2019, 120, 12822–12831. [Google Scholar] [CrossRef]
- Tian, X.; Ji, Y.; Liang, Y.; Zhang, J.; Guan, L.; Wang, C. LINC00520 targeting miR-27b-3p regulates OSMR expression level to promote acute kidney injury development through the PI3K/AKT signaling pathway. J. Cell. Physiol. 2019, 234, 14221–14233. [Google Scholar] [CrossRef]
- Geng, X.; Xu, X.; Fang, Y.; Zhao, S.; Hu, J.; Xu, J.; Jia, P.; Ding, X.; Teng, J. Effect of Long Non-Coding RNA Growth Arrest-Specific 5 on Apoptosis in Renal Ischemia/Reperfusion Injury. Nephrology 2019, 24, 405–413. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, F.; Zhu, T.; Li, J.; Gu, D.; Jiang, W.; Lu, Y.; Zhou, D. Mechanism of Long Non-Coding RNA MALAT1 in Lipopolysaccharide-Induced Acute Kidney Injury Is Mediated by the MiR-146a/NF-ΚB Signaling Pathway. Int. J. Mol. Med. 2018, 41, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Liu, S.; Wen, J.; Xue, L.; Zhang, Y.; Yan, D.; Wang, G.; Liu, Z. Inhibition of maternally expressed gene 3 attenuated lipopolysaccharide-induced apoptosis through sponging miR-21 in renal tubular epithelial cells. J. Cell. Biochem. 2018, 119, 7800–7806. [Google Scholar] [CrossRef] [PubMed]
- Kölling, M.; Genschel, C.; Kaucsar, T.; Hübner, A.; Rong, S.; Schmitt, R.; Sörensen-Zender, I.; Haddad, G.; Kistler, A.; Seeger, H.; et al. Hypoxia-induced long non-coding RNA Malat1 is dispensable for renal ischemia/reperfusion-injury. Sci. Rep. 2018, 8, 3438. [Google Scholar] [CrossRef] [Green Version]
- M’Baya-Moutoula, E.; Louvet, L.; Molinié, R.; Guerrera, I.C.; Cerutti, C.; Fourdinier, O.; Nourry, V.; Gutierrez, L.; Morlière, P.; Mesnard, F.; et al. A multi-omics analysis of the regulatory changes induced by miR-223 in a monocyte/macrophage cell line. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, S.; Yang, F.; Xie, J.; Chen, J.; Li, Z. Diosmetin Alleviates Acute Kidney Injury by Promoting the TUG1/Nrf2/HO-1 Pathway in Sepsis Rats. Int. Immunopharmacol. 2020, 88, 106965. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Ma, Z. Long Noncoding RNA HOXA-AS2 Mediates MicroRNA-106b-5p to Repress Sepsis Engendered Acute Kidney Injury. J. Biochem. Mol. Toxicol. 2020, 34, E22453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, N.; Zhang, B.; Xu, S.B. Long Noncoding RNA TCONS_00016406 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Regulating the miR-687/PTEN Pathway. Front. Physiol. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Ma, L.; Fu, P. An Update of Long-Noncoding RNAs in Acute Kidney Injury. Front Physiol. 2022, 13, 849403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, K.; Luo, H.; Wu, C.; Yu, W.; Cheng, F. Novel lncRNA XLOC_032768 alleviates cisplatin-induced apoptosis and inflammatory response of renal tubular epithelial cells through TNF-α. Int. Immunopharmacol. 2020, 83, 106472. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, P.M.; Niu, Y.; García Córdoba, C.A.; Huang, X.R.; Yu, C.; Lan, H.Y. Long Non-Coding RNA LRNA9884 Promotes Acute Kidney Injury via Regulating NF-KB-Mediated Transcriptional Activation of MIF. Front Physiol. 2020, 11, 590027. [Google Scholar] [CrossRef]
- Chen, F.; Hu, Y.; Xie, Y.; Zhao, Z.; Ma, L.; Li, Z.; Tan, W. Total Glucosides of Paeony Alleviate Cell Apoptosis and Inflammation by Targeting the Long Noncoding RNA XIST/MicroRNA-124-3p/ITGB1 Axis in Renal Ischemia/Reperfusion Injury. Mediat. Inflamm. 2020, 2020, 8869511. [Google Scholar] [CrossRef]
- Haddad, G.; Kölling, M.; Wegmann, U.A.; Dettling, A.; Seeger, H.; Schmitt, R.; Soerensen-Zender, I.; Haller, H.; Kistler, A.D.; Dueck, A.; et al. Renal AAV2-Mediated Overexpression of Long Non-Coding RNA H19 Attenuates Ischemic Acute Kidney Injury through Sponging of MicroRNA-30a-5p. J. Am. Soc. Nephrol. 2021, 32, 323–341. [Google Scholar] [CrossRef]
- Tian, H.; Wu, M.; Zhou, P.; Huang, C.; Ye, C.; Wang, L. The Long Non-Coding RNA MALAT1 Is Increased in Renal Ischemia-Reperfusion Injury and Inhibits Hypoxia-Induced Inflammation. Ren. Fail. 2018, 40, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Li, D.; Shen, W.; Shen, X.; Liu, Y. LncRNA NEAT1 Promotes Hypoxia-Induced Renal Tubular Epithelial Apoptosis through Downregulating MiR-27a-3p. J. Cell Biochem. 2019, 120, 16273–16282. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-M.; Palanisamy, K.; Sun, K.-T.; Day, Y.-J.; Shu, K.-H.; Wang, I.-K.; Shyu, W.-C.; Chen, P.; Chen, Y.-L.; Li, C.-Y. RANTES mediates kidney ischemia reperfusion injury through a possible role of HIF-1α and LncRNA PRINS. Sci. Rep. 2016, 6, 18424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washino, S.; Hosohata, K.; Miyagawa, T. Roles Played by Biomarkers of Kidney Injury in Patients with Upper Urinary Tract Obstruction. Int. J. Mol. Sci. 2020, 21, 5490. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; An, C.; Tran, M.; Du, H.; Wang, P.; Zhou, D.; Wang, Y. Pharmacological Inhibition of STAT6 Ameliorates Myeloid Fibroblast Activation and Alternative Macrophage Polarization in Renal Fibrosis. Front. Immunol. 2021, 12, 73014. [Google Scholar] [CrossRef]
- Zhou, S.-G.; Zhang, W.; Ma, H.-J.; Guo, Z.-Y.; Xu, Y. Silencing of LncRNA TCONS_00088786 reduces renal fibrosis through miR-132. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 166–173. [Google Scholar] [PubMed]
- Sun, J.; Zhang, S.; Shi, B.; Zheng, D.; Shi, J. Transcriptome Identified lncRNAs Associated with Renal Fibrosis in UUO Rat Model. Front. Physiol. 2017, 8, 658. [Google Scholar] [CrossRef] [Green Version]
- Feng, M.; Tang, P.M.-K.; Huang, X.-R.; Sun, S.-F.; You, Y.-K.; Xiao, J.; Lv, L.-L.; Xu, A.-P.; Lan, H.-Y. TGF-β Mediates Renal Fibrosis via the Smad3-Erbb4-IR Long Noncoding RNA Axis. Mol. Ther. 2018, 26, 148–161. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.-Y.; Liu, X.-S.; Huang, X.-R.; Yu, X.-Q.; Lan, H.-Y. Diverse Role of TGF-β in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; He, Y.; Gan, Y.; Zhang, B.; Dai, G.; Ru, F.; Jiang, Z.; Chen, Z.; Chen, X. Long Non-coding RNA: An Emerging Contributor and Potential Therapeutic Target in Renal Fibrosis. Front. Genet. 2021, 12, 682904. [Google Scholar] [CrossRef]
- Cao, L.; Qin, P.; Zhang, J.; Qiao, H.; Shi, P.; Huo, H. LncRNA PVT1 Suppresses the Progression of Renal Fibrosis via Inactivation of TGF-β Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 3547–3557. [Google Scholar] [CrossRef]
- Xie, H.; Xue, J.-D.; Chao, F.; Jin, Y.-F.; Fu, Q. Long non-coding RNA-H19 antagonism protects against renal fibrosis. Oncotarget 2016, 7, 51473–51481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Peng, Y.; Liang, Z.; Yang, Y.; Zhang, J. A Negative Feedback Loop of H19/MiR-675/EGR1 Is Involved in Diabetic Nephropathy by Downregulating the Expression of the Vitamin D Receptor. J. Cell. Physiol. 2019, 234, 17505–17513. [Google Scholar] [CrossRef] [PubMed]
- Okuyan, H.M.; Dogan, S.; Terzi, M.Y.; Begen, M.A.; Turgut, F.H. Association of Serum LncRNA H19 Expression with Inflammatory and Oxidative Stress Markers and Routine Biochemical Parameters in Chronic Kidney Disease. Clin. Exp. Nephrol. 2021, 25, 522–530. [Google Scholar] [CrossRef]
- Lv, P.; Liu, H.; Ye, T.; Yang, X.; Duan, C.; Yao, X.; Li, B.; Tang, K.; Chen, Z.; Liu, J.; et al. XIST Inhibition Attenuates Calcium Oxalate Nephrocalcinosis-Induced Renal Inflammation and Oxidative Injury via the MiR-223/NLRP3 Pathway. Oxid. Med. Cell Longev. 2021, 2021, 1676152. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.Q.; Ren, Y.Q.; Zhang, L.; Sun, L.N.; Man, Y.L.; Wang, Z.K. Effects of Long Non-Coding RNA LINC00667 on Renal Tubular Epithelial Cell Proliferation, Apoptosis and Renal Fibrosis via the MiR-19b-3p/LINC00667/CTGF Signaling Pathway in Chronic Renal Failure. Cell. Signal. 2019, 54, 102–114. [Google Scholar] [CrossRef]
- Huang, P.; Gu, X.J.; Huang, M.Y.; Tan, J.H.; Wang, J. Down-Regulation of LINC00667 Hinders Renal Tubular Epithelial Cell Apoptosis and Fibrosis through MiR-34c. Clin. Transl. Oncol. 2021, 23, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yuan, Q.; Chen, Y.; Huang, Z.; Fang, X.; Zhang, H.; Peng, L.; Xiao, P. LncRNA ENST00000453774.1 Contributes to Oxidative Stress Defense Dependent on Autophagy Mediation to Reduce Extracellular Matrix and Alleviate Renal Fibrosis. J. Cell. Physiol. 2019, 234, 9130–9143. [Google Scholar] [CrossRef]
- Ding, B.; Ma, G.; Wang, Z.; Liang, W.; Gao, W. Mechanisms of Kidney Cell Pyroptosis in Chronic Kidney Disease and the Effects of Traditional Chinese Medicine. Evid. Based. Complement. Altern. Med. 2021, 2021, 1173324. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Hu, S.; Qin, W.; Shi, J.; Zeng, C.; Bao, H.; Liu, Z. Upregulated Long Noncoding RNA LOC105375913 Induces Tubulointerstitial Fibrosis in Focal Segmental Glomerulosclerosis. Sci. Rep. 2019, 9, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Han, R.; Shi, J.; Zhu, X.; Qin, W.; Zeng, C.; Bao, H.; Liu, Z. The long noncoding RNA LOC105374325 causes podocyte injury in individuals with focal segmental glomerulosclerosis. J. Biol. Chem. 2018, 293, 20227–20239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortajada, A.; Gutiérrez, E.; de Jorge, E.G.; Anter, J.; Segarra, A.; Espinosa, M.; Blasco, M.; Roman, E.; Marco, H.; Quintana, L.F.; et al. Elevated factor H–related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int. 2017, 92, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Gholaminejad, A.; Gheisari, Y.; Jalali, S.; Roointan, A. Comprehensive Analysis of IgA Nephropathy Expression Profiles: Identification of Potential Biomarkers and Therapeutic Agents. BMC Nephrol. 2021, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhou, Q.; Huang, X.; Yu, J.; Han, Q.; Nong, B.; Xiong, Y.; Liang, P.; Li, J.; Feng, M.; et al. Identification of Differentially Expressed Circulating Exosomal LncRNAs in IgA Nephropathy Patients. BMC Immunol. 2020, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Shi, J.; Zhao, Y.; Li, C. LncRNA PTTG3P Induced Aberrant Glycosylated IgA1 Production and B Cell Growth in IgA Nephropathy. Environ. Sci. Pollut. Res. Int. 2021, 28, 56606–56614. [Google Scholar] [CrossRef]
- Zuo, N.; Li, Y.; Liu, N.; Wang, L. Differentially expressed long non-coding RNAs and mRNAs in patients with IgA nephropathy. Mol. Med. Rep. 2017, 16, 7724–7730. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Zhao, Z.; Li, F.; Ji, F.; Wen, J. ICAM-1 related long noncoding RNA is associated with progression of IgA nephropathy and fibrotic changes in proximal tubular cells. Sci. Rep. 2022, 12, 9645. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.W.; Pan, M.; Ye, H.Y.; Zheng, Y.; Chen, Y.; Huang, W.W.; Xu, X.Y.; Zheng, S.B. Down-Regulation of the Long Non-Coding RNA XIST Ameliorates Podocyte Apoptosis in Membranous Nephropathy via the MiR-217-TLR4 Pathway. Exp. Physiol. 2019, 104, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-S.; Hsieh, H.-Y.; Shih, H.-M.; Sytwu, H.-K.; Wu, C.-C. Urinary Xist is a potential biomarker for membranous nephropathy. Biochem. Biophys. Res. Commun. 2014, 452, 415–421. [Google Scholar] [CrossRef]
- Couser, W.G. Primary Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Pi, P.; Yin, Q.; Xiao, L.; Luo, D. Long Non-Coding RNA Neat1 Triggers Renal Tubular Epithelial Cell Apoptosis via Activating BH3-Only Protein in Membranous Nephropathy. Autoimmunity 2021, 54, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ye, Z.; Xue, Z.; Wu, L.; Ouyang, Y.; Yao, C.; Cui, C.; Xu, N.; Ma, J.; Hou, G.; et al. Identification of Renal Long Non-coding RNA RP11-2B6.2 as a Positive Regulator of Type I Interferon Signaling Pathway in Lupus Nephritis. Front. Immunol. 2019, 10, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psarras, A.; Emery, P.; Vital, E.M. Type I Interferon-Mediated Autoimmune Diseases: Pathogenesis, Diagnosis and Targeted Therapy. Rheumatol. Oxf. 2017, 56, 1662–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.; Jia, L.; Tang, Z.; Zheng, Y. Long non-coding RNA MIR22HG promotes osteogenic differentiation of bone marrow mesenchymal stem cells via PTEN/ AKT pathway. Cell Death Dis. 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Y.-B.; Yang, Y.-L.; Chen, B.-P.; Wang, C.-X.; Li, R.-H.; Huang, D. LncRNA MALAT1 inhibits osteogenic differentiation of mesenchymal stem cells in osteoporosis rats through MAPK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4609–4617. [Google Scholar] [PubMed]
- Zhang, L.; Yang, C.; Chen, S.; Wang, G.; Shi, B.; Tao, X.; Zhou, L.; Zhao, J. Long Noncoding RNA DANCR Is a Positive Regulator of Proliferation and Chondrogenic Differentiation in Human Synovium-Derived Stem Cells. DNA Cell Biol. 2017, 36, 136–142. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, Y.; Xiao, Z.; Zhao, Y.; Chen, B.; Yang, B.; Xu, B.; Song, H.; Zou, Y.; Ma, X.; et al. LncRNA Neat1 mediates miR-124-induced activation of Wnt/β-catenin signaling in spinal cord neural progenitor cells. Stem Cell Res. Ther. 2019, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Gao, Y.; Shi, S.; Zhao, D.; Cao, H.; Fu, T.; Cai, X.; Xiao, J. LncRNA-AK137033 inhibits the osteogenic potential of adipose-derived stem cells in diabetic osteoporosis by regulating Wnt signaling pathway via DNA methylation. Cell Prolif. 2021, 55, e13174. [Google Scholar] [CrossRef]
- Eades, G.; Zhang, Y.-S.; Li, Q.-L.; Xia, J.-X.; Yao, Y.; Zhou, Q. Long non-coding RNAs in stem cells and cancer. World J. Clin. Oncol. 2014, 5, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Herreros-Villanueva, M.; Zhang, J.-S.; Koenig, A.; Abel, E.V.; Smyrk, T.C.; Bamlet, W.R.; De Narvajas, A.A.-M.; Gomez, T.S.; Simeone, D.M.; Bujanda, L.; et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis 2013, 2, e61. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Jiang, X.; Duan, L.; Xiong, Q.; Yuan, Y.; Liu, P.; Jiang, L.; Shen, Q.; Zhao, S.; Yang, C.; et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol. Cancer 2021, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-K.; Kafert-Kasting, S.; Thum, T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef]
- Sallustio, F.; De Benedictis, L.; Castellano, G.; Zaza, G.; Loverre, A.; Costantino, V.; Grandaliano, G.; Schena, F.P. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J. 2009, 24, 514–525. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Aloisi, A.; Toma, C.C.; Marulli, E.; Serino, G.; Cox, S.N.; De Palma, G.; Stasi, A.; Divella, C.; et al. Inhibin-A and Decorin Secreted by Human Adult Renal Stem/Progenitor Cells Through the TLR2 Engagement Induce Renal Tubular Cell Regeneration. Sci. Rep. 2017, 7, 8225. [Google Scholar] [CrossRef] [PubMed]
- Angelotti, M.L.; Ronconi, E.; Ballerini, L.; Peired, A.; Mazzinghi, B.; Sagrinati, C.; Parente, E.; Gacci, M.; Carini, M.; Rotondi, M.; et al. Characterization of Renal Progenitors Committed Toward Tubular Lineage and Their Regenerative Potential in Renal Tubular Injury. Stem Cells 2012, 30, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Lazzeri, E.; Angelotti, M.L.; Peired, A.; Conte, C.; Marschner, J.A.; Maggi, L.; Mazzinghi, B.; Lombardi, D.; Melica, M.E.; Nardi, S.; et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat. Commun. 2018, 9, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of Renal Progenitor Cells from Adult Human Kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosali, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and Characterization of Multipotent Progenitor Cells from the Bowman’s Capsule of Adult Human Kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Gramignoli, R.; Sallustio, F.; Widera, D.; Raschzok, N. Editorial: Tissue Repair and Regenerative Mechanisms by Stem/Progenitor Cells and Their Secretome. Front. Med. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallustio, F.; Stasi, A.; Curci, C.; Divella, C.; Picerno, A.; Franzin, R.; De Palma, G.; Rutigliano, M.; Lucarelli, G.; Battaglia, M.; et al. Renal progenitor cells revert LPS-induced endothelial-to-mesenchymal transition by secreting CXCL6, SAA4, and BPIFA2 antiseptic peptides. FASEB J. 2019, 33, 10753–10766. [Google Scholar] [CrossRef] [PubMed]
- Picerno, A.; Castellano, G.; Curci, C.; Kopaczka, K.; Stasi, A.; Pertosa, G.B.; Sabbà, C.; Gesualdo, L.; Gramignoli, R.; Sallustio, F. The Icarus Flight of Perinatal Stem and Renal Progenitor Cells Within Immune System. Front. Immunol. 2022, 13, 840146. [Google Scholar] [CrossRef]
- Curci, C.; Picerno, A.; Chaoul, N.; Stasi, A.; De De Palma, G.; Franzin, R.; Pontrelli, P.; Castellano, G.; Pertosa, G.B.; Macchia, L.; et al. Adult Renal Stem/Progenitor Cells Can Modulate T Regulatory Cells and Double Negative T Cells. Int. J. Mol. Sci. 2020, 22, 274. [Google Scholar] [CrossRef]
- Picerno, A.; Stasi, A.; Franzin, R.; Curci, C.; di Bari, I.; Gesualdo, L.; Sallustio, F. Why stem/progenitor cells lose their regenerative potential. World J. Stem Cells 2021, 13, 1714–1732. [Google Scholar] [CrossRef]
- Fico, A.; Fiorenzano, A.; Pascale, E.; Patriarca, E.J.; Minchiotti, G. Long non-coding RNA in stem cell pluripotency and lineage commitment: Functions and evolutionary conservation. Cell. Mol. Life Sci. 2019, 76, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Picerno, A.; Giannuzzi, F.; Curci, C.; De Palma, G.; Di Chiano, M.G.; Simone, S.; Franzin, R.; Gallone, A.; Di Lorenzo, V.F.; Stasi, A.; et al. The long non-coding RNA HOTAIR controls the self-renewal, cell senescence, and secretion of antiaging protein α-Klotho in human adult renal progenitor cells. Stem Cells 2022, 40, 963–975. [Google Scholar] [CrossRef]
- Hu, M.C.; Bian, A.; Neyra, J.; Zhan, M. Klotho, stem cells, and aging. Clin. Interv. Aging 2015, 10, 1233–1243. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zhou, B.; Li, H.; Huang, X.; Wu, Y.; Xing, C.; Yu, X.; Ji, Y. Long noncoding RNA HOTAIR promotes the self-renewal of leukemia stem cells through epigenetic silencing of p15. Exp. Hematol. 2018, 67, 32–40.e3. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, R.; Tu, L.; Liu, D. Long non-coding RNA HOTAIR promotes burn wound healing by regulating epidermal stem cells. Mol. Med. Rep. 2020, 22, 1811–1820. [Google Scholar] [CrossRef]
- Bhan, A.; Mandal, S.S. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2015, 1856, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.-Q.; Yallowitz, A.R.; Sun, H.; Dressler, G.R.; Wellik, D.M. A Hox-Eya-Pax Complex Regulates Early Kidney Developmental Gene Expression. Mol. Cell. Biol. 2007, 27, 7661–7668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amândio, A.R.; Necsulea, A.; Joye, E.; Mascrez, B.; Duboule, D. Hotair Is Dispensible for Mouse Development. PLOS Genet. 2016, 12, e1006232. [Google Scholar] [CrossRef] [Green Version]
- Brossa, A.; Papadimitriou, E.; Collino, F.; Incarnato, D.; Oliviero, S.; Camussi, G.; Bussolati, B. Role of CD133 Molecule in Wnt Response and Renal Repair. Stem Cells Transl. Med. 2018, 7, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yuri, S.; Kimura, H.; Yanagawa, N.; Hamon, M.; Hauser, P.; Zhao, L.; Jo, O.D.; Yanagawa, N. Comprehensive analysis of chromatin signature and transcriptome uncovers functional lncRNAs expressed in nephron progenitor cells. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2019, 1862, 58–70. [Google Scholar] [CrossRef]
- Aguilar, R.; Spencer, K.B.; Kesner, B.; Rizvi, N.F.; Badmalia, M.D.; Mrozowich, T.; Mortison, J.D.; Rivera, C.; Smith, G.F.; Burchard, J.; et al. Targeting Xist with compounds that disrupt RNA structure and X inactivation. Nature 2022, 604, 160–166. [Google Scholar] [CrossRef]
- Sallustio, F.; Gesualdo, L.; Pisignano, D. The Heterogeneity of Renal Stem Cells and Their Interaction with Bio- and Nano-materials. Adv. Exp. Med. Biol. 2019, 1123, 195–216. [Google Scholar] [CrossRef]
- Tabet, F.; Vickers, K.C.; Torres, L.F.C.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannuzzi, F.; Maiullari, S.; Gesualdo, L.; Sallustio, F. The Mission of Long Non-Coding RNAs in Human Adult Renal Stem/Progenitor Cells and Renal Diseases. Cells 2023, 12, 1115. https://doi.org/10.3390/cells12081115
Giannuzzi F, Maiullari S, Gesualdo L, Sallustio F. The Mission of Long Non-Coding RNAs in Human Adult Renal Stem/Progenitor Cells and Renal Diseases. Cells. 2023; 12(8):1115. https://doi.org/10.3390/cells12081115
Chicago/Turabian StyleGiannuzzi, Francesca, Silvia Maiullari, Loreto Gesualdo, and Fabio Sallustio. 2023. "The Mission of Long Non-Coding RNAs in Human Adult Renal Stem/Progenitor Cells and Renal Diseases" Cells 12, no. 8: 1115. https://doi.org/10.3390/cells12081115