Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives
Abstract
:1. Introduction
2. Human Definition of SIRS and MODS
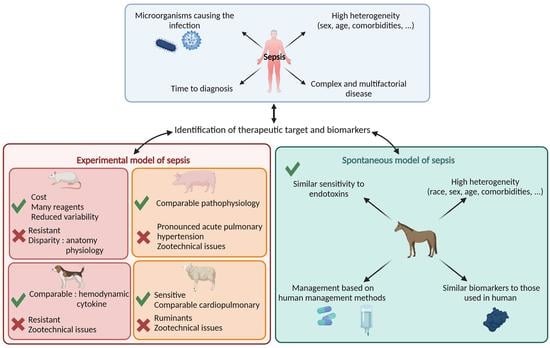
3. Preclinical Experimental Animal Models of Sepsis Benefits for Human Medicine
4. Spontaneous Equine Sepsis, a Pertinent Veterinary Disease for Modeling Human Sepsis
5. SIRS and MODS Definition for Adult Horse
5.1. Equine SIRS Definition
5.2. Equine MODS Definition
6. Main Biomarkers of Equine Sepsis: A Proof of Concept for Human Medicine
6.1. Main Biomarkers Currently Used to Diagnose Sepsis
6.1.1. Fibrinogen
6.1.2. D-Dimer
6.1.3. Serum Amyloid A
6.1.4. C-Reactive Protein
6.1.5. Procalcitonin
6.2. Clinical Perspective: Studying the Secretome to Identify New Biomarkers
7. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2015, 193, 259–272. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent Advances in Biosensors for Diagnosis and Detection of Sepsis: A Comprehensive Review. Biosens. Bioelectron. 2019, 124–125, 205–215. [Google Scholar] [CrossRef]
- Quenot, J.P.; Pavon, A.; Fournel, I.; Barbar, S.D.; Bruyère, R. Le choc septique de l’adulte en France: Vingt ans de données épidémiologiques. Réanimation 2015, 24, 303–309. [Google Scholar] [CrossRef]
- Monneret, G.; Gossez, M.; Aghaeepour, N.; Gaudilliere, B.; Venet, F. How Clinical Flow Cytometry Rebooted Sepsis Immunology. Cytom. A 2019, 95, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Teggert, A.; Datta, H.; Ali, Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines 2020, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Murando, F.; Peloso, A.; Cobianchi, L. Experimental Abdominal Sepsis: Sticking to an Awkward but Still Useful Translational Model. Mediat. Inflamm 2019, 2019, 8971036. [Google Scholar] [CrossRef]
- Blangy-Letheule, A.; Persello, A.; Rozec, B.; Waard, M.D.; Lauzier, B. New Approaches to Identify Sepsis Biomarkers: The Importance of Model and Sample Source for Mass Spectrometry. Oxid. Med. Cell. Longev. 2020, 2020, 6681073. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [Green Version]
- The Lancet Infectious Diseases. For Sepsis, the Drugs Don’t Work. Lancet Infect. Dis. 2012, 12, 89. [Google Scholar] [CrossRef]
- Guillon, A.; Preau, S.; Aboab, J.; Azabou, E.; Jung, B.; Silva, S.; Textoris, J.; Uhel, F.; Vodovar, D.; Zafrani, L.; et al. Preclinical Septic Shock Research: Why We Need an Animal ICU. Ann. Intensive Care 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, S.; Khan, M.; Liaw, P.; Fox-Robichaud, A. Experimental Sepsis Models; IntechOpen: London, UK, 2012; ISBN 978-953-51-0780-4. [Google Scholar]
- Nagy, S.; Tárnoky, K.; Tutsek, L.; Boros, M.; Karácsony, G. A Canine Model of Hyperdynamic Sepsis Induced by Intestinal Ischemia. Acta Physiol. Hung. 1990, 75, 303–320. [Google Scholar] [PubMed]
- Song, R.; Kim, J.; Yu, D.; Park, C.; Park, J. Kinetics of IL-6 and TNF-α Changes in a Canine Model of Sepsis Induced by Endotoxin. Vet. Immunol. Immunopathol. 2012, 146, 143–149. [Google Scholar] [CrossRef]
- Fink, M.P.; Heard, S.O. Laboratory Models of Sepsis and Septic Shock. J. Surg. Res. 1990, 49, 186–196. [Google Scholar] [CrossRef]
- Michie, H.R. The Value of Animal Models in the Development of New Drugs for the Treatment of the Sepsis Syndrome. J. Antimicrob. Chemother. 1998, 41, 47–49. [Google Scholar] [CrossRef]
- Ceciliani, F.; Restelli, L.; Lecchi, C. Proteomics in Farm Animals Models of Human Diseases. PROTEOMICS—Clin. Appl. 2014, 8, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Groenen, M.A.M.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Rogel-Gaillard, C.; Park, C.; Milan, D.; Megens, H.-J.; et al. Analyses of Pig Genomes Provide Insight into Porcine Demography and Evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Mair, K.H.; Sedlak, C.; Käser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The Porcine Innate Immune System: An Update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The Pig as an Animal Model for Human Pathologies: A Proteomics Perspective. PROTEOMICS—Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, E. Animal Models for Translational Proteomics. PROTEOMICS—Clin. Appl. 2014, 8, 637–639. [Google Scholar] [CrossRef]
- Cohen, R.I.; Hassell, A.-M.; Marzouk, K.; Marini, C.; Liu, S.F.; Scharf, S.M. Renal Effects of Nitric Oxide in Endotoxemia. Am. J. Respir. Crit. Care Med. 2001, 164, 1890–1895. [Google Scholar] [CrossRef]
- Ji, M.-H.; Yang, J.-J.; Wu, J.; Li, R.-Q.; Li, G.-M.; Fan, Y.-X.; Li, W.-Y. Experimental Sepsis in Pigs—Effects of Vasopressin on Renal, Hepatic, and Intestinal Dysfunction. Upsala J. Med. Sci. 2012, 117, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Maybauer, D.M.; Maybauer, M.O.; Szabó, C.; Cox, R.A.; Westphal, M.; Kiss, L.; Horvath, E.M.; Traber, L.D.; Hawkins, H.K.; Salzman, A.L.; et al. The peroxynitrite catalyst WW-85 improves pulmonary function in ovine septic shock. Shock 2011, 35, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traber, D.L.; Redl, H.; Schlag, G.; Herndon, D.N.; Kimura, R.; Prien, T.; Traber, L.D. Cardiopulmonary Responses to Continuous Administration of Endotoxin. Am. J. Physiol. Heart Circ. Physiol. 1988, 254, H833–H839. [Google Scholar] [CrossRef]
- Taylor, S. A Review of Equine Sepsis. Equine Vet. Educ. 2015, 27, 99–109. [Google Scholar] [CrossRef]
- McGovern, K.F.; Lascola, K.M.; Smith, S.A.; Clark-Price, S.C.; Wilkins, P.A.; Schaeffer, D.J.; Foreman, J.H. The Effects of Hyperglycemia and Endotoxemia on Coagulation Parameters in Healthy Adult Horses. J. Vet. Intern. Med. 2013, 27, 347–353. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Jacobs, C.C.; Cook, V.L.; Gandy, J.C.; Hauptman, J.G.; Sordillo, L.M. Duration of in Vivo Endotoxin Tolerance in Horses. Vet. Immunol. Immunopathol. 2016, 173, 10–16. [Google Scholar] [CrossRef]
- Scavone, D.; Sgorbini, M.; Borges, A.S.; Oliveira-Filho, J.P.; Vitale, V.; Paltrinieri, S. Serial Measurements of Paraoxonase-1 (PON-1) Activity in Horses with Experimentally Induced Endotoxemia. BMC Vet. Res. 2020, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Stasi, A.; Intini, A.; Gigante, M.; Di Palma, A.M.; Divella, C.; Netti, G.S.; Prattichizzo, C.; Pontrelli, P.; Crovace, A.; et al. Endothelial Dysfunction and Renal Fibrosis in Endotoxemia-Induced Oliguric Kidney Injury: Possible Role of LPS-Binding Protein. Crit. Care 2014, 18, 520. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.R.; Smagul, A.; Simpson, D.; Clegg, P.D.; Rubio-Martinez, L.M.; Peffers, M.J. The Synovial Fluid Proteome Differentiates between Septic and Nonseptic Articular Pathologies. J Proteom. 2019, 202, 103370. [Google Scholar] [CrossRef] [PubMed]
- Borde, L.; Amory, H.; Grulke, S.; Leroux, A.A.; Houben, R.M.; Detilleux, J.; Sandersen, C.C. Prognostic Value of Echocardiographic and Doppler Parameters in Horses Admitted for Colic Complicated by Systemic Inflammatory Response Syndrome. J. Vet. Emerg. Crit. Care 2014, 24, 302–310. [Google Scholar] [CrossRef] [Green Version]
- McConachie, E.; Giguère, S.; Barton, M.H. Scoring System for Multiple Organ Dysfunction in Adult Horses with Acute Surgical Gastrointestinal Disease. J. Vet. Intern. Med. 2016, 30, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Satué, K.; Gardon, J.C.; Muñoz, A. Clinical and Laboratorial Description of the Differential Diagnoses of Hemostatic Disorders in the Horse. Iran. J. Vet. Res. 2020, 21, 1–8. [Google Scholar]
- Senior, J.M.; Proudman, C.J.; Leuwer, M.; Carter, S.D. Plasma Endotoxin in Horses Presented to an Equine Referral Hospital: Correlation to Selected Clinical Parameters and Outcomes. Equine Vet. J. 2011, 43, 585–591. [Google Scholar] [CrossRef]
- Osterbur, K.; Mann, F.A.; Kuroki, K.; DeClue, A. Multiple Organ Dysfunction Syndrome in Humans and Animals. J. Vet. Intern. Med. 2014, 28, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.-F.; Kwong, G.P.S.; Lambert, J.; Massie, S.; Lockhart, S. Prognostic Value and Development of a Scoring System in Horses With Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2017, 31, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.N.; Vandenplas, M.L. Is It the Systemic Inflammatory Response Syndrome or Endotoxemia in Horses with Colic? Vet. Clin. N. Am. Equine Pract. 2014, 30, 337–351. [Google Scholar] [CrossRef]
- Schwarz, B.C.; van den Hoven, R.; Schwendenwein, I. Diagnostic Value of the Neutrophil Myeloperoxidase Index in Horses with Systemic Inflammation. Vet. J. 2012, 191, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.N.; Talavera, J.; Fernández Del Palacio, M.J. Usefulness of Doppler Ultrasonography to Assess Digital Vascular Dynamics in Horses with Systemic Inflammatory Response Syndrome or Laminitis. J. Am. Vet. Med. Assoc. 2013, 243, 1756–1761. [Google Scholar] [CrossRef]
- Epstein, K.L.; Brainard, B.M.; Giguere, S.; Vrono, Z.; Moore, J.N. Serial Viscoelastic and Traditional Coagulation Testing in Horses with Gastrointestinal Disease. J. Vet. Emerg. Crit. Care 2013, 23, 504–516. [Google Scholar] [CrossRef]
- Hoffman, A.M.; Staempfli, H.R.; Willan, A. Prognostic Variables for Survival of Neonatal Foals Under Intensive Care. J. Vet. Intern. Med. 1992, 6, 89–95. [Google Scholar] [CrossRef]
- Cohen, N.D.; Parson, E.M.; Seahorn, T.L.; Carter, G.K. Prevalence and Factors Associated with Development of Laminitis in Horses with Duodenitis/Proximal Jejunitis: 33 Cases (1985–1991). J. Am. Vet. Med. Assoc. 1994, 204, 250–254. [Google Scholar]
- Werners, A.H.; Bull, S.; Fink-Gremmels, J. Endotoxaemia: A Review with Implications for the Horse. Equine Vet. J. 2005, 37, 371–383. [Google Scholar] [CrossRef]
- Peek, S.F.; Semrad, S.; McGuirk, S.M.; Riseberg, A.; Slack, J.A.; Marques, F.; Coombs, D.; Lien, L.; Keuler, N.; Darien, B.J. Prognostic Value of Clinicopathologic Variables Obtained at Admission and Effect of Antiendotoxin Plasma on Survival in Septic and Critically Ill Foals. J. Vet. Intern. Med. 2006, 20, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Okabayashi, K.; Wada, H.; Ohta, S.; Shiku, H.; Nobori, T.; Maruyama, K. Hemostatic Markers and the Sepsis-Related Organ Failure Assessment Score in Patients with Disseminated Intravascular Coagulation in an Intensive Care Unit. Am. J. Hematol. 2004, 76, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Manocha, S.; Russell, J.A.; Sutherland, A.M.; Wattanathum, A.; Walley, K.R. Fibrinogen-Beta Gene Haplotype Is Associated with Mortality in Sepsis. J. Infect. 2007, 54, 572–577. [Google Scholar] [CrossRef]
- Garcia-Obregon, S.; Azkargorta, M.; Seijas, I.; Pilar-Orive, J.; Borrego, F.; Elortza, F.; Boyano, M.D.; Astigarraga, I. Identification of a Panel of Serum Protein Markers in Early Stage of Sepsis and Its Validation in a Cohort of Patients. J. Microbiol. Immunol. Infect. 2018, 51, 465–472. [Google Scholar] [CrossRef]
- Kibe, S.; Adams, K.; Barlow, G. Diagnostic and Prognostic Biomarkers of Sepsis in Critical Care. J. Antimicrob. Chemother. 2011, 66, ii33–ii40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, B.V.; Kold, S.E. Fibrinogen Response to Surgical Tissue Trauma in the Horse. Equine Vet. J. UK 1988, 20, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Crisman, M.V.; Scarratt, W.K.; Zimmerman, K.L. Blood Proteins and Inflammation in the Horse. Vet. Clin. N. Am Equine Pr. 2008, 24, 285–297. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and Sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Prasse, K.W.; Topper, M.J.; Moore, J.N.; Welles, E.G. Analysis of Hemostasis in Horses with Colic. J. Am. Vet. Med. Assoc. 1993, 203, 685–693. [Google Scholar]
- Topper, M.J.; Prasse, K.W. Analysis of Coagulation Proteins as Acute-Phase Reactants in Horses with Colic. Am. J. Vet. Res. 1998, 59, 542–545. [Google Scholar]
- Collatos, C.; Barton, M.H.; Prasse, K.W.; Moore, J.N. Intravascular and Peritoneal Coagulation and Fibrinolysis in Horses with Acute Gastrointestinal Tract Diseases. J. Am. Vet. Med. Assoc. 1995, 207, 465–470. [Google Scholar] [PubMed]
- Borges, A.S.; Divers, T.J.; Stokol, T.; Mohammed, O.H. Serum Iron and Plasma Fibrinogen Concentrations as Indicators of Systemic Inflammatory Diseases in Horses. J. Vet. Intern. Med. 2007, 21, 489–494. [Google Scholar] [CrossRef]
- Jacobsen, S. Review of Equine Acute-Phase Proteins. Infect. Dis. 2007, 53, 230–235. [Google Scholar]
- Jacobsen, S.; Andersen, P.H. The Acute Phase Protein Serum Amyloid A (SAA) as a Marker of Inflammation in Horses. Equine Vet. Educ. 2010, 19, 38–46. [Google Scholar] [CrossRef]
- Andrews, D.A.; Reagan, W.J.; DeNicola, D.B. Plasma Fibrinogen in Recognizing Equine Inflammatory Disease. Compend. Contin. Educ. Pract. Vet. USA 1994, 16, 1354–1366. [Google Scholar]
- Prinsen, B.H.C.M.T.; Rabelink, T.J.; Beutler, J.J.; Kaysen, G.A.; De Boer, J.; Boer, W.H.; Hagen, E.C.; Berger, R.; De Sain-Van Der Velden, M.G.M. Increased Albumin and Fibrinogen Synthesis Rate in Patients with Chronic Renal Failure. Kidney Int. 2003, 64, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Fiusa, M.M.L.; Carvalho-Filho, M.A.; Annichino-Bizzacchi, J.M.; de Paula, E.V. Causes and Consequences of Coagulation Activation in Sepsis: An Evolutionary Medicine Perspective. BMC Med. 2015, 13, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodelo, J.R.; de la Rosa, G.; Valencia, M.L.; Ospina, S.; Arango, C.M.; Gómez, C.I.; García, A.; Nuñez, E.; Jaimes, F.A. D-Dimer Is a Significant Prognostic Factor in Patients with Suspected Infection and Sepsis. Am. J. Emerg. Med. 2012, 30, 1991–1999. [Google Scholar] [CrossRef]
- Sandholm, M.; Vidovic, A.; Puotunen-Reinert, A.; Sankari, S.; Nyholm, K.; Rita, H. D-Dimer Improves the Prognostic Value of Combined Clinical and Laboratory Data in Equine Gastrointestinal Colic. Acta Vet. Scand. 1995, 36, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Monreal, L.; Anglés, A.; Espada, Y.; Monasterio, J.; Monreal, M. Hypercoagulation and Hypofibrinolysis in Horses with Colic and DIC. Equine Vet. J. 2000, 32, 19–25. [Google Scholar] [CrossRef]
- Stokol, T.; Erb, H.N.; De Wilde, L.; Tornquist, S.J.; Brooks, M. Evaluation of Latex Agglutination Kits for Detection of Fibrin(Ogen) Degradation Products and D-Dimer in Healthy Horses and Horses with Severe Colic. Vet. Clin. Pathol. 2005, 34, 375–382. [Google Scholar] [CrossRef]
- Delgado, M.A.; Monreal, L.; Armengou, L.; Ríos, J.; Segura, D. Peritoneal D-Dimer Concentration for Assessing Peritoneal Fibrinolytic Activity in Horses with Colic. J. Vet. Intern. Med. 2009, 23, 882–889. [Google Scholar] [CrossRef]
- Cesarini, C.; Monreal, L.; Armengou, L.; Delgado, M.á.; Ríos, J.; Jose-Cunilleras, E. Association of Admission Plasma D-Dimer Concentration with Diagnosis and Outcome in Horses with Colic. J. Vet. Intern. Med. 2010, 24, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.D.; Sun, L. Emerging Functions of Serum Amyloid A in Inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Tan, L. Serum Amyloid a Promotes Visfatin Expression in Macrophages. BioMed Res. Int. 2016, 2016, e4819327. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, M.L.; Moore, J.N.; Barton, M.H.; Roussel, A.J.; Cohen, N.D. Concentrations of Serum Amyloid A and Lipopolysaccharide-Binding Protein in Horses with Colic. Am. J. Vet. Res. 2005, 66, 1509–1516. [Google Scholar] [CrossRef]
- Jacobsen, S.; Nielsen, J.V.; Kjelgaard-Hansen, M.; Toelboell, T.; Fjeldborg, J.; Halling-Thomsen, M.; Martinussen, T.; Thoefner, M.B. Acute Phase Response to Surgery of Varying Intensity in Horses: A Preliminary Study. Vet. Surg. 2009, 38, 762–769. [Google Scholar] [CrossRef]
- Jacobsen, S.; Jensen, J.C.; Frei, S.; Jensen, A.L.; Thoefner, M.B. Use of Serum Amyloid A and Other Acute Phase Reactants to Monitor the Inflammatory Response after Castration in Horses: A Field Study. Equine Vet. J. 2005, 37, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.J.; Leise, B.S.; Burgess, B.A.; Morley, P.S.; Cloninger, M.; Hassel, D.M. Concentrations of Serum Amyloid A and Plasma Fibrinogen in Horses Undergoing Emergency Abdominal Surgery. J. Vet. Emerg. Crit. Care 2016, 26, 344–351. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.D.; Żmigrodzka, M.; Winnicka, A.; Miśkiewicz, A.; Strzelec, K.; Cywińska, A. Serum Amyloid A in Equine Health and Disease. Equine Vet. J. 2019, 51, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinovich, M.; Villarino, N.F.; Singer, E.; Robinson, C.S.; Rubio-Martínez, L.M. Can Blood Serum Amyloid A Concentrations in Horses Differentiate Synovial Sepsis from Extrasynovial Inflammation and Determine Response to Treatment? Vet. Rec. 2020, 187, 235. [Google Scholar] [CrossRef] [Green Version]
- Hultén, C.; Demmers, S. Serum Amyloid A (SAA) as an Aid in the Management of Infectious Disease in the Foal: Comparison with Total Leucocyte Count, Neutrophil Count and Fibrinogen. Equine Vet. J. 2002, 34, 693–698. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Zeller, J.; Potempa, L.A.; Pietersz, G.A.; Eisenhardt, S.U.; Peter, K. C-Reactive Protein and Its Structural Isoforms: An Evolutionary Conserved Marker and Central Player in Inflammatory Diseases and Beyond. Subcell. Biochem. 2020, 94, 499–520. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-Phase Proteins: As Diagnostic Tool. J. Pharm. Bioallied Sci 2011, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Camacho, C.; Losa, J. Biomarkers for Sepsis. Biomed Res. Int. 2014, 2014, 547818. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, M.J.; Franco-Martínez, L.; Martínez-Subiela, S.; Cerón, J.J. Biomarkers of Sepsis in Pigs, Horses and Cattle: From Acute Phase Proteins to Procalcitonin. Anim. Health Res. Rev. 2022, 23, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Fujinaga, T.; Naiki, M.; Mizuno, S.; Otomo, K. Isolation, Characterization, and Quantitative Analysis of C-Reactive Protein from Horses. Am. J. Vet. Res. 1990, 51, 1215–1220. [Google Scholar]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin in Sepsis and Systemic Inflammation: A Harmful Biomarker and a Therapeutic Target. Br. J. Pharmacol. 2010, 159, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High Serum Procalcitonin Concentrations in Patients with Sepsis and Infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Afsar, I.; Sener, A.G. Is Procalcitonin a Diagnostic and/or Prognostic Marker in Sepsis? Infect. Dis. Clin. Pract. 2015, 23, 3–6. [Google Scholar] [CrossRef]
- Rieger, M.; Kochleus, C.; Teschner, D.; Rascher, D.; Barton, A.K.; Geerlof, A.; Kremmer, E.; Schmid, M.; Hartmann, A.; Gehlen, H. A New ELISA for the Quantification of Equine Procalcitonin in Plasma as Potential Inflammation Biomarker in Horses. Anal. Bioanal. Chem. 2014, 406, 5507–5512. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Jose-Cunilleras, E.; Corazza, M.; Tognetti, R.; Guidi, G.; Intorre, L.; Sgorbini, M. Plasma Procalcitonin Concentration in Healthy Horses and Horses Affected by Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2015, 29, 1689–1691. [Google Scholar] [CrossRef]
- Teschner, D.; Rieger, M.; Koopmann, C.; Gehlen, H. Procalcitonin in horses with an acute colic. Pferdeheilkunde 2015, 31, 371–377. [Google Scholar] [CrossRef]
- Kilcoyne, I.; Nieto, J.E.; Dechant, J.E. Diagnostic Value of Plasma and Peritoneal Fluid Procalcitonin Concentrations in Horses with Strangulating Intestinal Lesions. J. Am. Vet. Med. Assoc. 2020, 256, 927–933. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Nocera, I.; Bonelli, F.; Vitale, V.; Meucci, V.; Conte, G.; Jose-Cunilleras, E.; Gracia-Calvo, L.A.; Sgorbini, M. Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses. Animals 2021, 11, 2015. [Google Scholar] [CrossRef]
- Parrillo, J.E.; Burch, C.; Shelhamer, J.H.; Parker, M.M.; Natanson, C.; Schuette, W. A Circulating Myocardial Depressant Substance in Humans with Septic Shock. Septic Shock Patients with a Reduced Ejection Fraction Have a Circulating Factor That Depresses in Vitro Myocardial Cell Performance. J. Clin. Investig. 1985, 76, 1539–1553. [Google Scholar] [CrossRef] [Green Version]
- Tjalsma, H.; Bolhuis, A.; Jongbloed, J.D.H.; Bron, S.; van Dijl, J.M. Signal Peptide-Dependent Protein Transport in Bacillus Subtilis: A Genome-Based Survey of the Secretome. Microbiol. Mol. Biol. Rev. 2000, 64, 515–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenau, J.; Michelland, S.; Seve, M. Le sécrétome: Définitions et intérêt biomédical. La Rev. Méd. Interne 2008, 29, 606–608. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- van Hees, H.W.; Schellekens, W.-J.M.; Linkels, M.; Leenders, F.; Zoll, J.; Donders, R.; Dekhuijzen, P.R.; van der Hoeven, J.G.; Heunks, L.M. Plasma from Septic Shock Patients Induces Loss of Muscle Protein. Crit. Care 2011, 15, R233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blangy-Letheule, A.; Persello, A.; Michelland, S.; Cunin, V.; Souab, F.; Aillerie, V.; Dhot, J.; Erraud, A.; Montnach, J.; Seve, M.; et al. The Secretome Deregulations in a Rat Model of Endotoxemic Shock. Oxid. Med. Cell. Longev. 2021, 2021, 6650464. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Salluh, J.I.F. Use of Biomarkers in Sepsis: Many Questions, Few Answers. Rev. Bras. Ter. Intensiv. 2013, 25, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, P.A. What’s in a Word? The Need for SIRS and Sepsis Definitions in Equine Medicine and Surgery. Equine Vet. J. 2018, 50, 7–9. [Google Scholar] [CrossRef] [PubMed]
| Calculation of the SOFA Score | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Respiration PaO2/FiO2 (kPa) | ≥53.3 | <53.3 | <40 | <26.7 with ventilation support | <13.3 with ventilation support |
| Coagulation Platelets (103/mm3) | >150 | <150 | <100 | <50 | <20 |
| Hepatic Bilirubin (µmol/L) | <20 | 20–32 | 33–101 | 102–204 | ≥204 |
| Cardiovascular | |||||
| Hypotension (mmHg) | MAP > 70 | MAP < 70 | |||
| Dopamine (µg/kg/min) | <5 | 5.1–15 | >15 | ||
| Central Nervous System Glasgow score | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Kidney | |||||
| Creatinine (µmol/L) | <110 | 110–170 | 171–299 | 300–400 | >440 |
| Urine (mL/day) | - | - | - | <500 | <500 |
| Models | Advantages | Disadvantages |
|---|---|---|
| Rodent | - Less expensive - Wide range of reagents - Wide range of transgenic model - Reduced variability - Short reproduction time | - Resistance to LPS - Zootechnical issues - Anatomy and physiology disparity with human |
| Canine | - Hemodynamic similarity - Cytokine similarity | - Resistance to LPS - Zootechnical issues - Long reproduction time |
| Sheep | - Diversity of races - Extremely sensitive to LPS - Cardiopulmonary similarity | - Ruminants - Zootechnical issues - Long reproduction time |
| Swine | - Omnivorous as humans - Anatomy and physiology similar to humans - Diversity of races - Reproduce the thermogenesis response to stress and the systemic energetic failure associated with septic shock | - Zootechnical issues - Long reproduction time - Pronounced acute pulmonary hypertension |
| Schwarz et al., 2012 | Aguirre et al., 2013 | Epstein et al., 2013 | Borde et al., 2014 | |
|---|---|---|---|---|
| Heart rate (Bpm) | >60 | >50 | ||
| Respiratory rate (Mpm) | >30 | >30 or PaCO2 < 32 mmHg | >30 | |
| Temperature (°C) | >38.5 | <37.2 or >38.5 | >38.6 | <36.7 or >38.6 |
| Cell counts of leukocytes (× 109 cells/L) | <5 or >10 | <4 or >12.5 or >10% immature GNN | <4.5 or >12.5 or >10% GNN immatures | <5 or >14.5 |
| Additional criteria | / | Lactate > 2 mmol/L or MAP < 90 mmHg | ||
| Clinical Sign | Threshold Values |
|---|---|
| Temperature (°C) | <37 or >38.5 |
| Heart rate (Bpm) | >52 |
| Respiratory rate (Mpm) | >20 or PaCO2 < 32 mmHg |
| Cell count of leukocytes (×109 cells/L) | <5 or >12.5 |
| Biomarkers | Advantages | Disadvantages |
|---|---|---|
| Fibrinogen | - Markers of inflammation - Inexpensive - Easy measurement | - Lack of specificity |
| Serum amyloid A | - Major acute phase proteins in horses - Distinguish between septic and non-septic inflammatory states - Rapid and pronounced increase in response to inflammatory disease | - Lack of specificity |
| Procalcitonin | - Increase markedly - Early marker of microbial infection | - Lack of specificity |
| Biomarkers | Human | Horses |
|---|---|---|
| Fibrinogen | -  or or  in sepsis [50,51] in sepsis [50,51]-  associated with mortality [50] associated with mortality [50] | -  in sepsis in sepsis-  24 h after induction of inflammation 24 h after induction of inflammation |
| D-dimer | -  in sepsis in sepsis | -  in sepsis in sepsis |
| C-reactive Protein | -  in sepsis in sepsis | -  in sepsis in sepsis |
| Serum amyloid A | -  in sepsis [52] in sepsis [52] | -  in sepsis in sepsis-  12 h after surgery 12 h after surgery |
| Procalcitonin | -  in septic patients in septic patients- Levels begin to rise 4 h after the onset of systemic infection and peak between 8 and 24 h [53] | -  in sepsis in sepsis- PCT remained higher in colic horses compared with healthy horses up to 96 h after admission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blangy-Letheule, A.; Vergnaud, A.; Dupas, T.; Rozec, B.; Lauzier, B.; Leroux, A.A. Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells 2023, 12, 1052. https://doi.org/10.3390/cells12071052
Blangy-Letheule A, Vergnaud A, Dupas T, Rozec B, Lauzier B, Leroux AA. Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells. 2023; 12(7):1052. https://doi.org/10.3390/cells12071052
Chicago/Turabian StyleBlangy-Letheule, Angélique, Amandine Vergnaud, Thomas Dupas, Bertrand Rozec, Benjamin Lauzier, and Aurélia A. Leroux. 2023. "Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives" Cells 12, no. 7: 1052. https://doi.org/10.3390/cells12071052