Effect of Remote Ischaemic Preconditioning on Perioperative Endothelial Dysfunction in Non-Cardiac Surgery: A Randomised Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
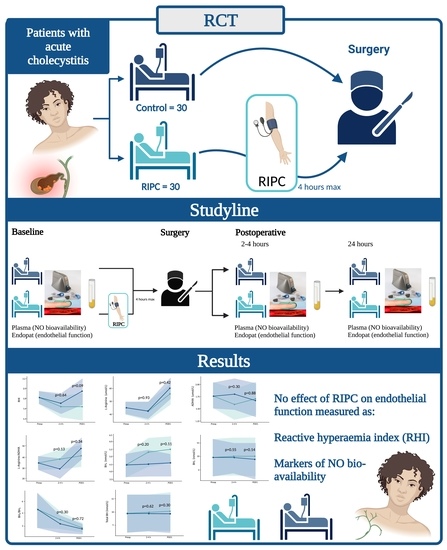
2.1. Trial Design and Setting
2.2. Participants
2.3. Randomisation and Blinding
2.4. Intervention
2.5. Outcomes
2.6. Data Sources
2.7. Statistical Analyses
3. Results
3.1. Patients
3.2. The Effect of RIPC on Endothelial Function and Nitric Oxide Bioavailability
3.3. The Effect of Surgery on Endothelial Function and Nitric Oxide Bioavailability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semel, M.; Lipsitz, S.; Funk, L.; Bader, A.; Weiser, T.; Gawande, A. Rates and patterns of death after surgery in the United States, 1996 and 2006. Surgery 2012, 151, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, P.J.; Goldman, L.; Cook, D.J.; Gilbert, K.; Leslie, K.; Guyatt, G.H. Perioperative cardiac events in patients undergoing noncardiac surgery: A review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005, 173, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaddaj Mallat, R.; Mathew John, C.; Kendrick, D.J.; Braun, A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017, 54, 458–470. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Benjamin, E.J. Assessment of Endothelial Function Using Digital Pulse Amplitude Tonometry. Trends Cardiovasc. Med. 2009, 19, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Lobysheva, I.I.; Biller, P.; Gallez, B.; Beauloye, C.; Balligand, J.L. Nitrosylated hemoglobin levels in human venous erythrocytes correlate with vascular endothelial function measured by digital reactive hyperemia. PLoS ONE 2013, 8, e76457. [Google Scholar] [CrossRef] [Green Version]
- Al Suwaidi, J.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shechter, M.; Issachar, A.; Marai, I.; Koren-Morag, N.; Freinark, D.; Shahar, Y.; Shechter, A.; Feinberg, M.S. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int. J. Cardiol. 2009, 134, 52–58. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Shechter, M.; Shechter, A.; Koren-Morag, N.; Feinberg, M.S.; Hiersch, L. Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. Am. J. Cardiol. 2014, 113, 162–167. [Google Scholar] [CrossRef]
- Ekeloef, S.; Oreskov, J.O.; Falkenberg, A.; Burcharth, J.; Schou-Pedersen, A.M.; Lykkesfeldt, J.; Gögenur, I. Endothelial dysfunction and myocardial injury after major emergency abdominal surgery: A prospective cohort study. BMC Anesthesiol. 2020, 20, 67. [Google Scholar] [CrossRef] [Green Version]
- Gokce, N.; Keaney, J.F.; Hunter, L.M.; Watkins, M.T.; Menzoian, J.O.; Vita, J.A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002, 105, 1567–1572. [Google Scholar] [CrossRef] [Green Version]
- Gokce, N.; Keaney, J.; Hunter, L.; Watkins, M.; Nedeljkovic, Z.; Menzoian, J.; Vita, J. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J. Am. Coll. Cardiol. 2003, 41, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlroy, D.; Chan, M.; Wallace, S.; Symons, J.; Koo, E.; Chu, L.; Myles, P. Automated preoperative assessment of endothelial dysfunction and risk stratification for perioperative myocardial injury in patients undergoing non-cardiac surgery. Br. J. Anaesth. 2014, 112, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Vascular Events In Noncardiac Surgery patients cOhort Evaluation (VISION) Writing Group on behalf of TV events I noncardiac S patIents cOhort evaluatioN (VISION) I. Myocardial Injury after Noncardiac Surgery A Large, International, Prospective Cohort Study Establishing Diagnostic Criteria, Characteristics, Predictors, and 30-day Outcomes. Anesthesiology 2014, 120, 564–578. [Google Scholar]
- Ekeloef, S.; Alamili, M.; Devereaux, P.J.; Gögenur, I. Troponin elevations after non-cardiac, non-vascular surgery are predictive of major adverse cardiac events and mortality: A systematic review and meta-analysis. Br. J. Anaesth. 2016, 117, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Hietala, P.; Strandberg, M.; Kiviniemi, T.; Strandberg, N.; Airaksinen, K.E.J. Usefulness of troponin T to predict short-term and long-term mortality in patients after hip fracture. Am. J. Cardiol. 2014, 114, 193–197. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.-S.; Xu, M.; Wen, S.-H.; Yao, X.; Wu, Y.; Huang, C.-Y.; Huang, W.-Q.; Liu, K.-X. Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: A randomized controlled trial. Anesthesiology 2013, 118, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.-N.; Wang, L.-R.; Wang, W.-T.; Jin, L.-L.; Zhao, X.-Y.; Zheng, L.-P.; Jin, L.-D.; Jiang, L.-M.; Xiong, X.-Q. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia-reperfusion. Anesth. Analg. 2010, 111, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Koca, K.; Yurttas, Y.; Cayci, T.; Bilgic, S.; Kaldirim, U.; Durusu, M.; Cekli, Y.; Ozkan, H.; Hanci, V.; Purtuloglu, T.; et al. The role of preconditioning and n-acetylcysteine on oxidative stress resulting from tourniquet-induced ischemia-reperfusion in arthroscopic knee surgery. J. Trauma-Inj. Infect. Crit. Care 2011, 70, 717–723. [Google Scholar] [CrossRef]
- Van, M.; Olguner, C.; Koca, U.; Şişman, A.R.; Muratli, K.; Karci, A.; Mavioğlu, M.; Kilercik, H. Ischaemic preconditioning attenuates haemodynamic response and lipid peroxidation in lower-extremity surgery with unilateral pneumatic tourniquet application: A clinical pilot study. Adv. Ther. 2008, 25, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, M.; Wu, Y.; Li, Y.-S.S.; Huang, W.-Q.Q.; Liu, K.-X.X. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: A randomized controlled study. Anesthesiology 2014, 121, 249–259. [Google Scholar] [CrossRef]
- Ekeloef, S.; Homilius, M.; Stilling, M.; Ekeloef, P.; Koyuncu, S.; Münster, A.-M.B.; Meyhoff, C.S.; Gundel, O.; Holst-Knudsen, J.; Mathiesen, O.; et al. The effect of remote ischaemic preconditioning on myocardial injury in emergency hip fracture surgery (PIXIE trial): Phase II randomised clinical trial. BMJ 2019, 367, l6395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukkar, L.; Hong, D.; Wong, M.G.; Badve, S.; Rogers, K.; Perkovic, V.; Walsh, M.; Yu, X.; Hillis, G.S.; Gallagher, M.; et al. Effects of ischaemic conditioning on major clinical outcomes in people undergoing invasive procedures: Systematic review and meta-analysis. BMJ (Clin. Res. Ed.) 2016, 355, i5599. [Google Scholar] [CrossRef] [Green Version]
- Wahlstrøm, K.L.; Bjerrum, E.; Gögenur, I.; Burcharth, J.; Ekeloef, S. Effect of remote ischaemic preconditioning on mortality and morbidity after non-cardiac surgery: Meta-analysis. Br. J. Surg. Open 2021, 5, zraa026. [Google Scholar] [CrossRef]
- Lang, J.A.; Kim, J.; Lang, J.A.; Kim, J. Remote ischaemic preconditioning-translating cardiovascular benefits to humans. J. Physiol. 2022, 600, 3053–3067. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2011, 9, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loukogeorgakis, S.P.; Panagiotidou, A.T.; Broadhead, M.W.; Donald, A.; Deanfield, J.E.; MacAllister, R.J. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: Role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005, 46, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Franke, W.D.; Lang, J.A. Delayed window of improvements in skin microvascular function following a single bout of remote ischaemic preconditioning. Exp. Physiol. 2021, 106, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Gantzer, J. eNOS and BH4. endothelial function or dysfunction.Importance of tetrahydrobiopterin. J. Neurol. Clin. Neurosci. 2018, 2, 1–4. [Google Scholar]
- McCrea, C.E.; Skulas-Ray, A.C.; Chow, M.; West, S.G. Test–retest reliability of pulse amplitude tonometry measures of vascular endothelial function: Implications for clinical trial design. Vasc. Med. 2012, 17, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Mortensen, A.; Lykkesfeldt, J. Kinetics of acid-induced degradation of tetra- and dihydrobiopterin in relation to their relevance as biomarkers of endothelial function. Biomark. Indic. Expo. Response Susceptibility Chem. 2013, 18, 55–62. [Google Scholar] [CrossRef]
- Fukushima, T.; Nixon, J.C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 1980, 102, 176–188. [Google Scholar] [CrossRef]
- Schou-Pedersen, A.M.V.; Lykkesfeldt, J. Comparison of Three Sample Preparation Procedures for the Quantification of L-Arginine, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Human Plasma Using HPLC-FLD. J. Anal. Methods Chem. 2018, 2018, 6148515. [Google Scholar] [CrossRef] [Green Version]
- Ozenne, B.; Forman, J. LMMstar: Repeated Measurement Models for Discrete Times. 2021. Available online: https://cran.r-project.org/web/packages/LMMstar/LMMstar.pdf (accessed on 15 March 2023).
- RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. Available online: http://www.rstudio.com/ (accessed on 15 March 2023).
- Ekeloef, S.; Gundel, O.; Falkenberg, A.; Mathiesen, O.; Gögenur, I. The effect of remote ischaemic preconditioning on endothelial function after hip fracture surgery. Acta Anaesthesiol. Scand. 2021, 65, 169–175. [Google Scholar] [CrossRef]
- Manchurov, V.; Ryazankina, N.; Khmara, T.; Skrypnik, D.; Reztsov, R.; Vasilieva, E.; Shpektor, A. Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am. J. Med. 2014, 127, 670–673. [Google Scholar] [CrossRef]
- Kharbanda, R.K.; Peters, M.; Walton, B.; Kattenhorn, M.; Mullen, M.; Klein, N.; Vallance, P.; Deanfield, J.; MacAllister, R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 2001, 103, 1624–1630. [Google Scholar] [CrossRef] [Green Version]
- Rytter, N.; Carter, H.; Piil, P.; Sørensen, H.; Ehlers, T.; Holmegaard, F.; Tuxen, C.; Jones, H.; Thijssen, D.; Gliemann, L.; et al. Ischemic Preconditioning Improves Microvascular Endothelial Function in Remote Vasculature by Enhanced Prostacyclin Production. J. Am. Heart Assoc. 2020, 9, 016017. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; Pedone, C.; Mondi, A.; Nunziata, E. Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis 2011, 219, 750–752. [Google Scholar] [CrossRef]
- Münzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Wang, Z.; Koeth, R.; Levison, B.; DelFraino, B.; Dzavik, V.; Griffith, O.W.; Hathaway, D.; Panza, J.A.; Nissen, S.E.; et al. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation 2007, 116, 2315–2324. [Google Scholar] [CrossRef] [Green Version]
- Böger, R.H.; Bode-Böger, S.M.; Thiele, W.; Junker, W.; Alexander, K.; Frölich, J.C. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 1997, 95, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabtree, M.J.; Smith, C.L.; Lam, G.; Goligorsky, M.S.; Gross, S.S. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1530–H1540. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Kar, S.; Kavdia, M. Computational analysis of interactions of oxidative stress and tetrahydrobiopterin reveals instability in eNOS coupling. Microvasc. Res. 2017, 114, 114–128. [Google Scholar] [CrossRef]
- Dewitte, K.; Claeys, M.; Van Craenenbroeck, E.; Monsieurs, K.; Heidbuchel, H.; Hoymans, V.; Stoop, T. Role of oxidative stress, angiogenesis and chemo-attractant cytokines in the pathogenesis of ischaemic protection induced by remote ischaemic conditioning: Study of a human model of ischaemia-reperfusion induced vascular injury. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2019, 26, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ekeloef, S.; Godthaab, C.; Schou-Pedersen, A.M.v.; Lykkesfeldt, J.; Gögenur, I. Peri-operative endothelial dysfunction in patients undergoing minor abdominal surgery: An observational study. Eur. J. Anaesthesiol. 2019, 36, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Ekeloef, S.; Larsen, M.H.H.; Schou-Pedersen, A.M.v.; Lykkesfeldt, J.; Rosenberg, J.; Gögenür, I. Endothelial dysfunction in the early postoperative period after major colon cancer surgery. Br. J. Anaesth. 2017, 118, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, S.; Kohjitani, A.; Miyata, M.; Tohya, A.; Yamashita, K.; Hashiguchi, T.; Ohishi, M.; Sugimura, M. Recovery of Endothelial Function after Minor-to-Moderate Surgery Is Impaired by Diabetes Mellitus, Obesity, Hyperuricemia and Sevoflurane-Based Anesthesia. Int. Heart J. 2018, 59, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Kottenberg, E.; Thielmann, M.; Bergmann, L.; Heine, T.; Jakob, H.; Heusch, G.; Peters, J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—A clinical trial. Acta Anaesthesiol. Scand. 2012, 56, 30–38. [Google Scholar] [CrossRef]
- Cho, Y.J.; Nam, K.; Kim, T.K.; Choi, S.W.; Kim, S.J.; Hausenloy, D.J.; Jeon, Y. Sevoflurane, propofol and carvedilol block myocardial protection by limb remote ischemic preconditioning. Int. J. Mol. Sci. 2019, 20, 269. [Google Scholar] [CrossRef] [Green Version]



| Baseline Characteristics | RIPC (n = 30) | Control (n = 30) | p-Value |
|---|---|---|---|
| Sex, No. (%) | |||
| Males | 20 (67) | 17 (57) | 0.43 |
| Age, Median (range); years | 54 (29–80) | 49 (33–77) | 0.24 |
| Body Mass Index, median (range); kg/m2 | 29.7 (19.7–41.5) | 29.3 (19.6–48.0) | 0.89 |
| Daily smoking, No. (%) | 8 (27) | 7 (23) | 0.77 |
| Alcohol abuse, No. (%) | 1 (3) | 2 (7) | 0.55 |
| Comorbidity, A No. (%) | |||
| Hypertension | 8 (27) | 5 (17) | 0.35 |
| Hypercholesterolemia | 2 (7) | 3 (10) | 0.64 |
| Diabetes mellitus | 2 (7) | 3 (10) | 0.64 |
| Ischemic heart disease | 1 (3) | 0 (0) | 0.97 |
| Atrial fibrillation | 2 (7) | 0 (0) | 0.49 |
| Previous Stroke | 2 (7) | 2 (7) | 1.0 |
| Daily Medicine intake, No. (%) | |||
| Beta-blocker | 1 (3) | 0 (0) | 0.97 |
| Calcium antagonist | 4 (13) | 1 (3) | 0.16 |
| ACE-1/ARB | 3 (10) | 5 (17) | 0.45 |
| NSAID | 4 (13) | 4 (13) | 1.0 |
| Glucocorticoids | 0 (0) | 2 (7) | 0.49 |
| Opioid | 3 (10) | 4 (13) | 0.69 |
| ASA classification, No. (%) | 0.40 | ||
| ASA I | 11 (18) | 10 (17) | |
| ASA II | 14 (23) | 18 (30) | |
| ASA III | 5 (8) | 2 (3) | |
| Preoperative medication, No. (%) | |||
| Antibiotics | 21 (70) | 21 (70) | 1.0 |
| Opioid | 3 (10) | 4 (13) | 0.69 |
| NSAID | 4 (13) | 4 (13) | 1.0 |
| Symptomatic days | |||
| Prior to surgery, median (range) | 3 (1–5) | 3 (1–5) | 0.82 |
| Surgery | |||
| Duration, min, median (range) | 96 (48–274) | 110 (44–240) | 0.82 |
| Blood loss, mL, median (range) | 150 (20–1200) | 250 (20–1550) | 0.18 |
| Bile leakage, No. (%) | 18 (60) | 13 (43) | 0.20 |
| Propofol, mg, median (range) | 985 (220–1400) | 1022 (399–1550) | 0.77 |
| Analgesia 24 h post-operative | |||
| Acetaminophen | |||
| No. (%) | 12 (40) | 8 (27) | 0.27 |
| mg, median (range) | 1 (1.0–2.0) | 1 (1.0–3.0) | |
| NSAID | |||
| No. (%) | 5 (17) | 5 (17) | 1.0 |
| mg, median (range) | 1000 (400–1600) | 800 (200–1200) | 0.47 |
| Morphine | |||
| No. (%) | 2 (3) | 6 (20) | 0.13 |
| mg, median (range) | 7.5 (5.0–10.0) | 15.0 (5.0–45.0) | 0.08 |
| Clavien–Dindo Classification, No. (%) | 0.80 | ||
| 1 | 1 (3) | 1 (3) | |
| 2 | 7 (23) | 5 (17) | |
| 3a | 2 (7) | 0 (0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahlstrøm, K.L.; Hansen, H.F.; Kvist, M.; Burcharth, J.; Lykkesfeldt, J.; Gögenur, I.; Ekeloef, S. Effect of Remote Ischaemic Preconditioning on Perioperative Endothelial Dysfunction in Non-Cardiac Surgery: A Randomised Clinical Trial. Cells 2023, 12, 911. https://doi.org/10.3390/cells12060911
Wahlstrøm KL, Hansen HF, Kvist M, Burcharth J, Lykkesfeldt J, Gögenur I, Ekeloef S. Effect of Remote Ischaemic Preconditioning on Perioperative Endothelial Dysfunction in Non-Cardiac Surgery: A Randomised Clinical Trial. Cells. 2023; 12(6):911. https://doi.org/10.3390/cells12060911
Chicago/Turabian StyleWahlstrøm, Kirsten L., Hannah F. Hansen, Madeline Kvist, Jakob Burcharth, Jens Lykkesfeldt, Ismail Gögenur, and Sarah Ekeloef. 2023. "Effect of Remote Ischaemic Preconditioning on Perioperative Endothelial Dysfunction in Non-Cardiac Surgery: A Randomised Clinical Trial" Cells 12, no. 6: 911. https://doi.org/10.3390/cells12060911