PDE6D Mediates Trafficking of Prenylated Proteins NIM1K and UBL3 to Primary Cilia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmid Construction
2.3. Generation of Stable Cell Lines
2.4. Affinity Proteomics
2.5. MS Analysis, Protein Quantification and Statistics
2.6. Visible Immunoprecipitation (VIP) Assay
2.7. Yeast Two-Hybrid (Y2H) Assay
2.8. Immunofluorescence Microscopy
2.9. Animals
2.10. Sub-Retinal Injections and Electroporation
2.11. Dark and Light Adaptation
2.12. Retinal Immunohistochemistry
2.13. Ultrastructure Expansion Microscopy (U-ExM) on Mouse Retina
3. Results
3.1. Tandem Affinity Proteomics of PDE6D Reveals a Strong Association to Prenylated (Ciliary) Proteins
3.2. PDE6D Strongly Interacts with Prenylated FAM219A, NIM1K, and UBL3
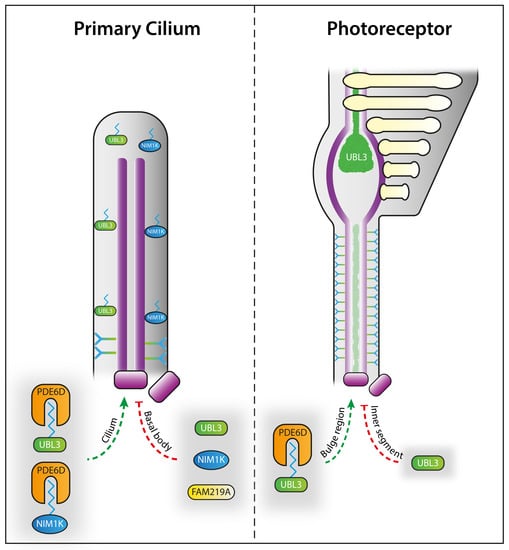
3.3. Ciliary Proteins FAM219A, NIM1K, and UBL3 Localize in a Prenylation-Dependent Manner
3.4. Affinity Proteomics of UBL3 Reveals Its Association to sEVs and Ciliogenesis
3.5. UBL3 Localizes to Specific Photoreceptor Compartments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nachury, M.V.; Mick, D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Blacque, O.E.; Leroux, M.R. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012, 13, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, H.; Marshall, W.F. Ciliogenesis: Building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 2011, 12, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.R.; Corbit, K.C.; Sirerol-Piquer, M.S.; Ramaswami, G.; Otto, E.A.; Noriega, T.R.; Seol, A.D.; Robinson, J.F.; Bennett, C.L.; Josifova, D.J.; et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 2011, 43, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, X.H.; Zhang, K.; Chen, C.K.; Frederick, J.M.; Prestwich, G.D.; Baehr, W. Photoreceptor cGMP phosphodiesterase δ subunit (PDEδ) functions as a prenyl-binding protein. J. Biol. Chem. 2004, 279, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinensky, M.M. Recent advances in the study of prenylated proteins. Biochim. Et Biophys. Acta 2000, 1484, 93–106. [Google Scholar] [CrossRef]
- Resh, M.D. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2006, 2, 584–590. [Google Scholar] [CrossRef]
- Norton, A.W.; Hosier, S.; Terew, J.M.; Li, N.; Dhingra, A.; Vardi, N.; Baehr, W.; Cote, R.H. Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J. Biol. Chem. 2005, 280, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fansa, E.K.; Wittinghofer, A. Sorting of lipidated cargo by the Arl2/Arl3 system. Small GTPases 2016, 7, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.L. Membrane trafficking: Arls squeeze the fat out. Nat. Chem. Biol. 2011, 7, 863–864. [Google Scholar] [CrossRef]
- Thomas, S.; Wright, K.J.; Le Corre, S.; Micalizzi, A.; Romani, M.; Abhyankar, A.; Saada, J.; Perrault, I.; Amiel, J.; Litzler, J.; et al. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum. Mutat. 2014, 35, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megarbane, A.; Hmaimess, G.; Bizzari, S.; El-Bazzal, L.; Al-Ali, M.T.; Stora, S.; Delague, V.; El-Hayek, S. A novel PDE6D mutation in a patient with Joubert syndrome type 22 (JBTS22). Eur. J. Med. Genet. 2019, 62, 103576. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Kowal, T.J.; Ning, K.; Koo, E.B.; Wu, A.Y.; Mahajan, V.B.; Sun, Y. Review of Ocular Manifestations of Joubert Syndrome. Genes 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.H. Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc. Natl. Acad. Sci. USA 2007, 104, 8857–8862. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. Mutations in the RPGR gene cause X-linked cone dystrophy. Hum. Mol. Genet. 2002, 11, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Roosing, S.; Rohrschneider, K.; Beryozkin, A.; Sharon, D.; Weisschuh, N.; Staller, J.; Kohl, S.; Zelinger, L.; Peters, T.A.; Neveling, K.; et al. Mutations in RAB28, encoding a farnesylated small GTPase, are associated with autosomal-recessive cone-rod dystrophy. Am. J. Hum. Genet. 2013, 93, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Roosing, S.; Collin, R.W.; den Hollander, A.I.; Cremers, F.P.; Siemiatkowska, A.M. Prenylation defects in inherited retinal diseases. J. Med. Genet. 2014, 51, 143–151. [Google Scholar] [CrossRef]
- Salinas, R.Y.; Pearring, J.N.; Ding, J.D.; Spencer, W.J.; Hao, Y.; Arshavsky, V.Y. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J. Cell Biol. 2017, 216, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Ageta, H.; Ageta-Ishihara, N.; Hitachi, K.; Karayel, O.; Onouchi, T.; Yamaguchi, H.; Kahyo, T.; Hatanaka, K.; Ikegami, K.; Yoshioka, Y.; et al. UBL3 modification influences protein sorting to small extracellular vesicles. Nat. Commun. 2018, 9, 3936. [Google Scholar] [CrossRef] [Green Version]
- Causier Barry, B. Analysing protein-protein interactions with the yeast two-hybrid system. Plant Mol. Biol. 2002, 50, 855–870. [Google Scholar] [CrossRef]
- Gloeckner, C.J.; Boldt, K.; Schumacher, A.; Roepman, R.; Ueffing, M. A novel tandem affinity purification strategy for the efficient isolation and characterisation of native protein complexes. Proteomics 2007, 7, 4228–4234. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.; Kampinga, H.H. Computational analysis of the human HSPH/HSPA/DNAJ family and cloning of a human HSPH/HSPA/DNAJ expression library. Cell Stress Chaperones 2009, 14, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell Robert, E.R. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 7877–7882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldt, K.; van Reeuwijk, J.; Lu, Q.; Koutroumpas, K.; Nguyen, T.M.; Texier, Y.; van Beersum, S.E.; Horn, N.; Willer, J.R.; Mans, D.A.; et al. An organelle-specific protein landscape identifies novel diseases and molecular mechanisms. Nat. Commun. 2016, 7, 11491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, T.; Klose, F.; Kuret, A.; Hoffmann, F.; Lukowski, R.; Ueffing, M.; Boldt, K. Tissue- and isoform-specific protein complex analysis with natively processed bait proteins. J. Proteom. 2021, 231, 103947. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [Green Version]
- Palsuledesai, C.C.; Distefano, M.D. Protein prenylation: Enzymes, therapeutics, and biotechnology applications. ACS Chem. Biol. 2015, 10, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Katoh, Y.; Nakamura, K.; Nakayama, K. Visible Immunoprecipitation (VIP) Assay: A Simple and Versatile Method for Visual Detection of Protein-protein Interactions. Bio-Protocol 2018, 8, e2687. [Google Scholar] [CrossRef]
- Mercey, O.; Kostic, C.; Bertiaux, E.; Giroud, A.; Sadian, Y.; Gaboriau, D.C.A.; Morrison, C.G.; Chang, N.; Arsenijevic, Y.; Guichard, P.; et al. The connecting cilium inner scaffold provides a structural foundation that protects against retinal degeneration. PLoS Biol. 2022, 20, e3001649. [Google Scholar] [CrossRef]
- Fansa, E.K.; O’Reilly, N.J.; Ismail, S.; Wittinghofer, A. The N- and C-terminal ends of RPGR can bind to PDE6delta. EMBO Rep. 2015, 16, 1583–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, B.M.; Schmidt, A.; Behrmann, E.; Burger, J.; Mielke, T.; Spahn, C.M.T.; Heck, M.; Scheerer, P. Mechanistic insights into the role of prenyl-binding protein PrBP/δ in membrane dissociation of phosphodiesterase 6. Nat. Commun. 2018, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.N. Expression and characterization of human PDEδ and its Caenorhabditis elegans ortholog CEδ. FEBS Lett. 1998, 440, 454–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linari, M.M. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc. Natl. Acad. Sci. USA 1999, 96, 1315–1320. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.; Fogelgren, B.; Lipschutz, J.H. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J. Biol. Chem. 2011, 286, 22469–22477. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Chacon-Heszele, M.F.; Huang, L.; McKenna, S.; Wilson, F.P.; Zuo, X.; Lipschutz, J.H. Cdc42 deficiency causes ciliary abnormalities and cystic kidneys. J. Am. Soc. Nephrol. 2013, 24, 1435–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.Y.; Baek, J.I.; Zuo, X.; Kim, S.H.; Dunaief, J.L.; Lipschutz, J.H. Cdc42 and sec10 Are Required for Normal Retinal Development in Zebrafish. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3361–3370. [Google Scholar] [CrossRef] [Green Version]
- Dyson, J.M.; Conduit, S.E.; Feeney, S.J.; Hakim, S.; DiTommaso, T.; Fulcher, A.J.; Sriratana, A.; Ramm, G.; Horan, K.A.; Gurung, R.; et al. INPP5E regulates phosphoinositide-dependent cilia transition zone function. J. Cell Biol. 2017, 216, 247–263. [Google Scholar] [CrossRef] [Green Version]
- Kosling, S.K.; Fansa, E.K.; Maffini, S.; Wittinghofer, A. Mechanism and dynamics of INPP5E transport into and inside the ciliary compartment. Biol. Chem. 2018, 399, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.S.; Gerstner, C.D.; Cady, M.A.; Arshavsky, V.Y.; Mitchell, C.; Ying, G.; Frederick, J.M.; Baehr, W. Deletion of the phosphatase INPP5E in the murine retina impairs photoreceptor axoneme formation and prevents disc morphogenesis. J. Biol. Chem. 2021, 296, 100529. [Google Scholar] [CrossRef]
- Razafsky, D.; Ward, C.; Potter, C.; Zhu, W.; Xue, Y.; Kefalov, V.J.; Fong, L.G.; Young, S.G.; Hodzic, D. Lamin B1 and lamin B2 are long-lived proteins with distinct functions in retinal development. Mol. Biol. Cell 2016, 27, 1928–1937. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.R.; You, L.R.; Wang, W.J.; Huang, W.S.; Chu, C.T.; Chi, Y.H.; Chen, H.C. Lamin A-mediated nuclear lamina integrity is required for proper ciliogenesis. EMBO Rep. 2020, 21, e49680. [Google Scholar] [CrossRef] [PubMed]
- Babbey, C.M.; Bacallao, R.L.; Dunn, K.W. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol.-Renal Physiol. 2010, 299, F495–F506. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.S.; Chua, C.E.; Tang, B.L. Rabs and other small GTPases in ciliary transport. Biol. Cell 2011, 103, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ochi, Y.; Satoh, T.; Satoh, A.K. Rab10, Crag and Ehbp1 regulate the basolateral transport of Na+K+ATPase in Drosophila photoreceptors. J. Cell Sci. 2020, 133, jcs238790. [Google Scholar] [CrossRef] [PubMed]
- Akella, J.S.; Carter, S.P.; Nguyen, K.; Tsiropoulou, S.; Moran, A.L.; Silva, M.; Rizvi, F.; Kennedy, B.N.; Hall, D.H.; Barr, M.M.; et al. Ciliary Rab28 and the BBSome negatively regulate extracellular vesicle shedding. eLife 2020, 9, e50580. [Google Scholar] [CrossRef]
- Wang, G.; Hu, H.B.; Chang, Y.; Huang, Y.; Song, Z.Q.; Zhou, S.B.; Chen, L.; Zhang, Y.C.; Wu, M.; Tu, H.Q.; et al. Rab7 regulates primary cilia disassembly through cilia excision. J. Cell Biol. 2019, 218, 4030–4041. [Google Scholar] [CrossRef] [Green Version]
- Gakovic, M.; Shu, X.; Kasioulis, I.; Carpanini, S.; Moraga, I.; Wright, A.F. The role of RPGR in cilia formation and actin stability. Hum. Mol. Genet. 2011, 20, 4840–4850. [Google Scholar] [CrossRef] [Green Version]
- Megaw, R.D.; Soares, D.C.; Wright, A.F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 2015, 138, 32–41. [Google Scholar] [CrossRef]
- Dutta, N.; Seo, S. RPGR, a prenylated retinal ciliopathy protein, is targeted to cilia in a prenylation- and PDE6D-dependent manner. Biol. Open 2016, 5, 1283–1289. [Google Scholar] [CrossRef]
- Kim, S.O.; Cho, K.S.; Kim, B.Y.; Lee, K.H. Cullin 1 (CUL1) Promotes Primary Ciliogenesis through the Induction of Ubiquitin-Proteasome-Dependent Dvl2 Degradation. Int. J. Mol. Sci. 2021, 22, 7572. [Google Scholar] [CrossRef] [PubMed]
- May-Simera, H.L.; Wan, Q.; Jha, B.S.; Hartford, J.; Khristov, V.; Dejene, R.; Chang, J.; Patnaik, S.; Lu, Q.; Banerjee, P.; et al. Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells. Cell Rep. 2018, 22, 189–205. [Google Scholar] [CrossRef] [Green Version]
- Florio, S.K.; Prusti, R.K.; Beavo, J.A. Solubilization of membrane-bound rod phosphodiesterase by the rod phosphodiesterase recombinant delta subunit. J. Biol. Chem. 1996, 271, 24036–24047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.C.; Weihbrecht, K.; Searby, C.C.; Li, Y.; Pope, R.M.; Sheffield, V.C.; Seo, S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc. Natl. Acad. Sci. USA 2012, 109, 19691–19696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maza, N.A.; Schiesser, W.E.; Calvert, P.D. An intrinsic compartmentalization code for peripheral membrane proteins in photoreceptor neurons. J. Cell Biol. 2019, 218, 3753–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fansa, E.K.; Kosling, S.K.; Zent, E.; Wittinghofer, A.; Ismail, S. PDE6delta-mediated sorting of INPP5E into the cilium is determined by cargo-carrier affinity. Nat. Commun. 2016, 7, 11366. [Google Scholar] [CrossRef] [Green Version]
- Martin-Morales, R.; Cilleros-Rodriguez, D.; Barbeito, P.; Deb Roy, A.; Loukil, A.; Sierra-Rodero, B.; Herranz, G.; Pampliega, O.; Redrejo-Rodriguez, M.; Goetz, S.C.; et al. Multiple ciliary localization signals control INPP5E ciliary targeting. eLife 2022, 11, e78383. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Cao, M.; Ning, J.; Hernandez-Lara, C.I.; Belzile, O.; Wang, Q.; Dutcher, S.K.; Liu, Y.; Snell, W.J. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. eLife 2015, 4, e05242. [Google Scholar] [CrossRef] [PubMed]
- Nager, A.R.; Goldstein, J.S.; Herranz-Perez, V.; Portran, D.; Ye, F.; Garcia-Verdugo, J.M.; Nachury, M.V. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 2017, 168, 252–263.e14. [Google Scholar] [CrossRef] [Green Version]
- Volz, A.K.; Frei, A.; Kretschmer, V.; de Jesus Domingues, A.M.; Ketting, R.F.; Ueffing, M.; Boldt, K.; Kramer-Albers, E.M.; May-Simera, H.L. Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nat. Commun. 2021, 12, 5671. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhang, F.; Xu, N.; Liu, G.; Diener, D.R.; Rosenbaum, J.L.; Huang, K. Comparative Analysis of Ciliary Membranes and Ectosomes. Curr. Biol. 2016, 26, 3327–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phua, S.C.; Chiba, S.; Suzuki, M.; Su, E.; Roberson, E.C.; Pusapati, G.V.; Schurmans, S.; Setou, M.; Rohatgi, R.; Reiter, J.F.; et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 2017, 168, 264–279.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat. Commun. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, M.; Ratnayake, I.; Janga, M.; Fogarty, E.; Scheidt, S.; Grassmeyer, J.; deRiso, J.; Chandrasekar, I.; Ahrenkiel, P.; Kopan, R.; et al. Notch signaling regulates Akap12 expression and primary cilia length during renal tubule morphogenesis. FASEB J. 2020, 34, 9512–9530. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Mason, H.A.; Cepko, C.L. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development 2006, 133, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Emerson, M.M. Notch signaling represses cone photoreceptor formation through the regulation of retinal progenitor cell states. Sci. Rep. 2021, 11, 14525. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Yin, Y.; Jang, S.C.; Lasser, C.; Wennmalm, S.; Hoffmann, H.J.; Li, L.; Gho, Y.S.; Nilsson, J.A.; Lotvall, J. Endosomal signalling via exosome surface TGFβ-1. J. Extracell. Vesicles 2019, 8, 1650458. [Google Scholar] [CrossRef]
- Baehr, W. Membrane protein transport in photoreceptors: The function of PDEdelta: The Proctor lecture. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8653–8666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, G.; Boldt, K.; Ueffing, M.; Gerstner, C.D.; Frederick, J.M.; Baehr, W. The small GTPase Rab28 is required for phagocytosis of cone outer segments by the murine retinal pigmented epithelium. J. Biol. Chem. 2018, 293, 17546–17558. [Google Scholar] [CrossRef] [PubMed]







| Plasmid | N-Tag | Purpose | Reference |
|---|---|---|---|
| pBD-GAL4_Cam/DEST | pBD | DNA-binding domain Y2H | [20] |
| pAD-GAL4_2.1/DEST | pAD | Activating domain Y2H | [20] |
| SF-TAP/N-TAP | Tandem StrepII/FLAG | Tandem affinity Purification | [21] |
| pcDNA5/FRT/TO GFP | eGFP | Immunofluorescence/VIP assay/electroporation | [22] |
| pDest-733 | mRFP | Immunofluorescence/VIP assay | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faber, S.; Letteboer, S.J.F.; Junger, K.; Butcher, R.; Tammana, T.V.S.; van Beersum, S.E.C.; Ueffing, M.; Collin, R.W.J.; Liu, Q.; Boldt, K.; et al. PDE6D Mediates Trafficking of Prenylated Proteins NIM1K and UBL3 to Primary Cilia. Cells 2023, 12, 312. https://doi.org/10.3390/cells12020312
Faber S, Letteboer SJF, Junger K, Butcher R, Tammana TVS, van Beersum SEC, Ueffing M, Collin RWJ, Liu Q, Boldt K, et al. PDE6D Mediates Trafficking of Prenylated Proteins NIM1K and UBL3 to Primary Cilia. Cells. 2023; 12(2):312. https://doi.org/10.3390/cells12020312
Chicago/Turabian StyleFaber, Siebren, Stef J. F. Letteboer, Katrin Junger, Rossano Butcher, Trinadh V. Satish Tammana, Sylvia E. C. van Beersum, Marius Ueffing, Rob W. J. Collin, Qin Liu, Karsten Boldt, and et al. 2023. "PDE6D Mediates Trafficking of Prenylated Proteins NIM1K and UBL3 to Primary Cilia" Cells 12, no. 2: 312. https://doi.org/10.3390/cells12020312