Cytotoxic CD8+ T Cells Are Involved in the Thrombo-Inflammatory Response during First-Diagnosed Atrial Fibrillation
Abstract
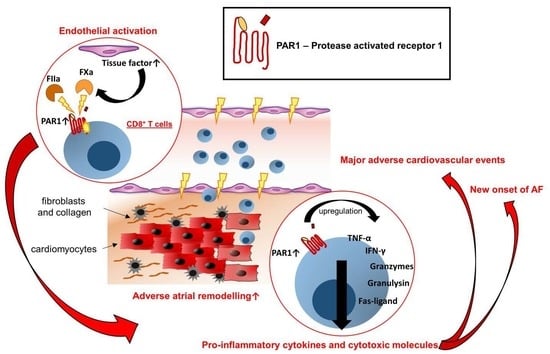
:1. Introduction
2. Materials and Methods
2.1. Patient Studies
2.1.1. Patients with First-Diagnosed AF and Control Group
2.1.2. Patients with Paroxysmal AF (Endomyocardial Biopsy)
2.1.3. Patients on Anti-Thrombotic Therapy after First Diagnosis of AF
2.2. ELISA
2.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Activation of CD8+ T Cells in Patients with First-Diagnosed AF
3.2. Activation of CD8+ T Cells Correlates with Biomarkers of Cardiac Fibrosis and Atrial Dysfunction in Patients with First-Diagnosed AF
3.3. CD8+ T Cell-Mediated Effector Function in Early AF Is Linked to PAR1 Activation
3.4. Frequency of Thrombin-Activated CD8+PAR1+ T Cells Is Associated with Adverse Outcomes in Patients with First Diagnosis of AF
4. Discussion
- The activation of CD8+ T cells is present in patients with FDAF.
- Cardiac fibrosis and atrial dysfunction correlate with CD8+ T cell activation.
- CD8+ T cell-mediated effector function in early AF is linked to PAR1 activation.
- The frequency of thrombin-activated CD8+PAR1+ T cells is associated with adverse outcomes in patients with FDAF.
- PAR-directed therapeutics (FIIa/FXa-inhibitors) mediate pleiotropic effects by targeting T cell effector function.
4.1. Activation of CD8+ T Cells in Patients with First-Diagnosed AF
4.2. CD8+ T Cell-Mediated Effector Function in Early AF Is Linked to PAR1 Activation
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Goette, A.; Lendeckel, U. Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences. Cells 2021, 10, 2605. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Borof, K.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Kuck, K.-H.; Wegscheider, K.; Kirchhof, P. Presenting Pattern of Atrial Fibrillation and Outcomes of Early Rhythm Control Therapy. J. Am. Coll. Cardiol. 2022, 80, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Hammwöhner, M.; Bukowska, A.; Mahardika, W.; Goette, A. Clinical importance of atrial cardiomyopathy. Int. J. Cardiol. 2019, 287, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. EP Eur. 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Ten Cate, H.; Guzik, T.J.; Eikelboom, J.; Spronk, H.M.H. Pleiotropic actions of factor Xa inhibition in cardiovascular prevention: Mechanistic insights and implications for anti-thrombotic treatment. Cardiovasc. Res. 2021, 117, 2030–2044. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, E.; Becker, C.; Bergmeier, W.; Bode, C.; Bourne, J.H.; Brown, H.; Buller, H.R.; Ten Cate-Hoek, A.J.; Ten Cate, V.; van Cauteren, Y.J.M.; et al. Thrombo-Inflammation in Cardiovascular Disease: An Expert Consensus Document from the Third Maastricht Consensus Conference on Thrombosis. Thromb. Haemost. 2020, 120, 538–564. [Google Scholar] [CrossRef] [Green Version]
- Bukowska, A.; Zacharias, I.; Weinert, S.; Skopp, K.; Hartmann, C.; Huth, C.; Goette, A. Coagulation factor Xa induces an inflammatory signalling by activation of protease-activated receptors in human atrial tissue. Eur. J. Pharmacol. 2013, 718, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Bukowska, A.; Hammwöhner, M.; Corradi, D.; Mahardhika, W.; Goette, A. Atrial thrombogenesis in atrial fibrillation: Results from atrial fibrillation models and AF-patients. Herzschrittmacherther. Elektrophysiol. 2018, 29, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Posma, J.J.; Posthuma, J.J.; Spronk, H.M. Coagulation and non-coagulation effects of thrombin. J. Thromb. Haemost. 2016, 14, 1908–1916. [Google Scholar] [CrossRef]
- Spronk, H.M.H.; de Jong, A.M.; Crijns, H.J.; Schotten, U.; Van Gelder, I.C.; ten Cate, H. Pleiotropic effects of factor Xa and thrombin: What to expect from novel anticoagulants. Cardiovasc. Res. 2014, 101, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posma, J.J.; Grover, S.P.; Hisada, Y.; Owens, A.P., 3rd; Antoniak, S.; Spronk, H.M.; Mackman, N. Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chandrabalan, A.; Ramachandran, R. Molecular mechanisms regulating Proteinase-Activated Receptors (PARs). FEBS J. 2021, 288, 2697–2726. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R.; De Ceunynck, K. Targeting PAR1: Now What? Trends Pharmacol. Sci. 2017, 38, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Nieman, M.T.; Kerlin, B.A. Protease-activated receptors: An illustrated review. Res. Pract. Thromb. Haemost. 2021, 5, 17–26. [Google Scholar] [CrossRef]
- Han, X.; Nieman, M.T. The domino effect triggered by the tethered ligand of the protease activated receptors. Thromb. Res. 2020, 196, 87–98. [Google Scholar] [CrossRef]
- López, M.L.; Soriano-Sarabia, N.; Bruges, G.; Marquez, M.E.; Preissner, K.T.; Schmitz, M.L.; Hackstein, H. Expression pattern of protease activated receptors in lymphoid cells. Cell. Immunol. 2014, 288, 47–52. [Google Scholar] [CrossRef]
- Friebel, J.; Moritz, E.; Witkowski, M.; Jakobs, K.; Strässler, E.; Dörner, A.; Steffens, D.; Puccini, M.; Lammel, S.; Glauben, R.; et al. Pleiotropic Effects of the Protease-Activated Receptor 1 (PAR1) Inhibitor, Vorapaxar, on Atherosclerosis and Vascular Inflammation. Cells 2021, 10, 3517. [Google Scholar] [CrossRef]
- Friebel, J.; Weithauser, A.; Witkowski, M.; Rauch, B.H.; Savvatis, K.; Dörner, A.; Tabaraie, T.; Kasner, M.; Moos, V.; Bösel, D.; et al. Protease-activated receptor 2 deficiency mediates cardiac fibrosis and diastolic dysfunction. Eur. Heart J. 2019, 40, 3318–3332. [Google Scholar] [CrossRef]
- Friebel, J.; Witkowski, M.; Rauch, U. Treating the Unstable Atherosclerotic Plaque by Targeting Activated Factor X– Anticoagulation and Beyond. Circ. J. 2015, 79, 2329–2331. [Google Scholar] [CrossRef]
- Malz, R.; Weithauser, A.; Tschope, C.; Schultheiss, H.P.; Rauch, U. Inhibition of coagulation factor Xa improves myocardial function during CVB3-induced myocarditis. Cardiovasc. Ther. 2014, 32, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.; Owens, A.P., 3rd; Baunacke, M.; Williams, J.C.; Lee, R.D.; Weithauser, A.; Sheridan, P.A.; Malz, R.; Luyendyk, J.P.; Esserman, D.A.; et al. PAR-1 contributes to the innate immune response during viral infection. J. Clin. Investig. 2013, 123, 1310–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weithauser, A.; Bobbert, P.; Antoniak, S.; Bohm, A.; Rauch, B.H.; Klingel, K.; Savvatis, K.; Kroemer, H.K.; Tschope, C.; Stroux, A.; et al. Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J. Am. Coll. Cardiol. 2013, 62, 1737–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weithauser, A.; Rauch, U. Role of protease-activated receptors (PARs) for the innate immune response of the heart. Trends Cardiovasc. Med. 2014, 24, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, W. Rauch. Tissue Factor—A link between vascular procoagulability and inflammation. Exp. Clin. Cardiol. 2014, 20, 5297–5303. [Google Scholar]
- Alice, W.; Marco, W.; Ursula, R. The Role of Protease-Activated Receptors for the Development of Myocarditis: Possible Therapeutic Implications. Curr. Pharm. Des. 2016, 22, 472–484. [Google Scholar] [CrossRef]
- Santos-Zas, I.; Lemarié, J.; Zlatanova, I.; Cachanado, M.; Seghezzi, J.C.; Benamer, H.; Goube, P.; Vandestienne, M.; Cohen, R.; Ezzo, M.; et al. Cytotoxic CD8(+) T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat. Commun. 2021, 12, 1483. [Google Scholar] [CrossRef]
- Leistner, D.M.; Kränkel, N.; Meteva, D.; Abdelwahed, Y.S.; Seppelt, C.; Stähli, B.E.; Rai, H.; Skurk, C.; Lauten, A.; Mochmann, H.C.; et al. Differential immunological signature at the culprit site distinguishes acute coronary syndrome with intact from acute coronary syndrome with ruptured fibrous cap: Results from the prospective translational OPTICO-ACS study. Eur. Heart J. 2020, 41, 3549–3560. [Google Scholar] [CrossRef]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8(+)CD28(-) Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef] [Green Version]
- Hurley, A.; Smith, M.; Karpova, T.; Hasley, R.B.; Belkina, N.; Shaw, S.; Balenga, N.; Druey, K.M.; Nickel, E.; Packard, B.; et al. Enhanced effector function of CD8(+) T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J. Infect. Dis. 2013, 207, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Smith, M.; Herz, J.; Li, T.; Hasley, R.; Le Saout, C.; Zhu, Z.; Cheng, J.; Gronda, A.; Martina, J.A.; et al. The role of protease-activated receptor 1 signaling in CD8 T cell effector functions. iScience 2021, 24, 103387. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Chen, Y.; Liu, Z.; Wang, Y.; Ge, W.; Kang, Y.; Guo, S. The PD-1 with PD-L1 Axis Is Pertinent with the Immune Modulation of Atrial Fibrillation by Regulating T Cell Excitation and Promoting the Secretion of Inflammatory Factors. J. Immunol. Res. 2022, 2022, 3647817. [Google Scholar] [CrossRef]
- Liu, L.; Lee, J.; Fu, G.; Liu, X.; Wang, H.; Zhang, Z.; Zheng, Q. Activation of peripheral blood CD3(+) T-lymphocytes in patients with atrial fibrillation. Int. Heart J. 2012, 53, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory signalling in atrial cardiomyocytes: A novel unifying principle in atrial fibrillation pathophysiology. Nat. Rev. Cardiol. 2022, 1–23. [Google Scholar] [CrossRef]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Schinnerling, K.; Moos, V.; Geelhaar, A.; Allers, K.; Loddenkemper, C.; Friebel, J.; Conrad, K.; Kühl, A.A.; Erben, U.; Schneider, T. Regulatory T cells in patients with Whipple’s disease. J. Immunol. 2011, 187, 4061–4067. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.S.; Probst, C.K.; Brazee, P.L.; Rotile, N.J.; Blasi, F.; Weinreb, P.H.; Black, K.E.; Sosnovik, D.E.; Van Cott, E.M.; Violette, S.M.; et al. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight 2017, 2, e86608. [Google Scholar] [CrossRef] [PubMed]
- Moos, V.; Feurle, G.E.; Schinnerling, K.; Geelhaar, A.; Friebel, J.; Allers, K.; Moter, A.; Kikhney, J.; Loddenkemper, C.; Kühl, A.A.; et al. Immunopathology of immune reconstitution inflammatory syndrome in Whipple’s disease. J. Immunol. 2013, 190, 2354–2361. [Google Scholar] [CrossRef] [Green Version]
- Merino-Merino, A.; Gonzalez-Bernal, J.; Fernandez-Zoppino, D.; Saez-Maleta, R.; Perez-Rivera, J.A. The Role of Galectin-3 and ST2 in Cardiology: A Short Review. Biomolecules 2021, 11, 1167. [Google Scholar] [CrossRef]
- Cunha, P.S.; Laranjo, S.; Heijman, J.; Oliveira, M.M. The Atrium in Atrial Fibrillation—A Clinical Review on How to Manage Atrial Fibrotic Substrates. Front. Cardiovasc. Med. 2022, 9, 1654. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Witkowski, M.; Friebel, J.; Buffa, J.A.; Li, X.S.; Wang, Z.; Sangwan, N.; Li, L.; DiDonato, J.A.; Tizian, C.; et al. Vascular endothelial Tissue Factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc. Res. 2021, 118, 2367–2384. [Google Scholar] [CrossRef] [PubMed]
- Hobbelt, A.H.; Spronk, H.M.; Crijns, H.J.G.M.; Ten Cate, H.; Rienstra, M.; Van Gelder, I.C. Prethrombotic State in Young Very Low-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 1990–1992. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Tong, S.; Xiang, Y.; Wu, L.; Xu, B.; Zhang, Y.; Ma, X.; Li, Y.; Song, Z.; Zhong, L. Association of hemostatic markers with atrial fibrillation: A meta-analysis and meta-regression. PLoS ONE 2015, 10, e0124716. [Google Scholar] [CrossRef] [PubMed]
- Negreva, M.N.; Prodanova, K.; Vitlianova, K.; Madjova, C. Paroxysmal atrial fibrillation: Changes in factor VIII and von Willebrand factor impose early hypercoagulability. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e140–e147. [Google Scholar] [CrossRef]
- Tóth, N.K.; Csanádi, Z.; Hajas, O.; Kiss, A.; Nagy-Baló, E.; Kovács, K.B.; Sarkady, F.; Muszbek, L.; Bereczky, Z.; Csiba, L.; et al. Intracardiac Hemostasis and Fibrinolysis Parameters in Patients with Atrial Fibrillation. Biomed. Res. Int. 2017, 2017, 3678017. [Google Scholar] [CrossRef] [Green Version]
- Shahid, F.; Lip, G.Y.H.; Shantsila, E. Role of Monocytes in Heart Failure and Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e007849. [Google Scholar] [CrossRef] [Green Version]
- Hammer, A.; Niessner, A.; Sulzgruber, P. The impact of CD4(+)CD28(null) T lymphocytes on atrial fibrillation: A potential pathophysiological pathway. Inflamm Res. 2021, 70, 1011–1014. [Google Scholar] [CrossRef]
- Sulzgruber, P.; Koller, L.; Winter, M.P.; Richter, B.; Blum, S.; Korpak, M.; Hülsmann, M.; Goliasch, G.; Wojta, J.; Niessner, A. The impact of CD4(+)CD28(null) T-lymphocytes on atrial fibrillation and mortality in patients with chronic heart failure. Thromb. Haemost. 2017, 117, 349–356. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, G.; Chen, X.; Li, Y.; Li, H.; Cheng, D.; Tang, Y.; Sang, H. IL-6-miR-210 Suppresses Regulatory T Cell Function and Promotes Atrial Fibrosis by Targeting Foxp3. Mol. Cells 2020, 43, 438–447. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, Y.; Liu, A.; Wu, Q.; Hu, Y.; Liu, J.; Zhu, H.; Yin, T.; Pan, D. Uncovering potential novel biomarkers and immune infiltration characteristics in persistent atrial fibrillation using integrated bioinformatics analysis. Math. Biosci. Eng. 2021, 18, 4696–4712. [Google Scholar] [CrossRef]
- Sulzgruber, P.; Thaler, B.; Koller, L.; Baumgartner, J.; Pilz, A.; Steininger, M.; Schnaubelt, S.; Fleck, T.; Laufer, G.; Steinlechner, B.; et al. CD4(+)CD28(null) T Lymphocytes are Associated with the Development of Atrial Fibrillation after Elective Cardiac Surgery. Sci. Rep. 2018, 8, 9624. [Google Scholar] [CrossRef] [Green Version]
- Kazem, N.; Sulzgruber, P.; Thaler, B.; Baumgartner, J.; Koller, L.; Laufer, G.; Steinlechner, B.; Hohensinner, P.; Wojta, J.; Niessner, A. CD8+CD28null T Lymphocytes are Associated with the Development of Atrial Fibrillation after Elective Cardiac Surgery. Thromb. Haemost. 2020, 120, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.; Pfister, R.; Mollenhauer, M.; Adler, C.; Kozlowski, J.; Wodarz, A.; Drebber, U.; Wippermann, J.; Michels, G. Inflammatory cell infiltration in left atrial appendageal tissues of patients with atrial fibrillation and sinus rhythm. Sci. Rep. 2020, 10, 1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Emmens, R.W.; van Wezenbeek, J.; Stooker, W.; Allaart, C.P.; Vonk, A.B.A.; van Rossum, A.C.; Niessen, H.W.M.; Krijnen, P.A.J. Atrial inflammation in different atrial fibrillation subtypes and its relation with clinical risk factors. Clin. Res. Cardiol. 2020, 109, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y.; Platonov, P.G. Histological evidence of inflammatory reaction associated with fibrosis in the atrial and ventricular walls in a case-control study of patients with history of atrial fibrillation. Europace 2016, 18, iv156–iv162. [Google Scholar] [CrossRef]
- Yamashita, T.; Sekiguchi, A.; Suzuki, S.; Ohtsuka, T.; Sagara, K.; Tanabe, H.; Kunihara, T.; Sawada, H.; Aizawa, T. Enlargement of the left atrium is associated with increased infiltration of immune cells in patients with atrial fibrillation who had undergone surgery. J. Arrhythm. 2015, 31, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, L.; Diederichsen, S.Z.; Haugan, K.J.; Brandes, A.; Graff, C.; Krieger, D.; Kronborg, C.; Køber, L.; Peters, D.C.; Olesen, M.S.; et al. Left Atrial Late Gadolinium Enhancement is Associated With Incident Atrial Fibrillation as Detected by Continuous Monitoring with Implantable Loop Recorders. JACC Cardiovasc. Imaging 2020, 13, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Ward, E.J.; Marelli-Berg, F.M. Mechanisms of T cell organotropism. Cell Mol. Life Sci. 2016, 73, 3009–3033. [Google Scholar] [CrossRef] [Green Version]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Alenezi, F.; Sun, J.L.; Alhanti, B.; Vaduganathan, M.; Oh, J.K.; Redfield, M.M.; Butler, J.; Hernandez, A.F.; Velazquez, E.J.; et al. Biomarker Profile of Left Atrial Myopathy in Heart Failure With Preserved Ejection Fraction: Insights From the RELAX Trial. J. Card. Fail. 2020, 26, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Lancini, D.; Greenslade, J.; Martin, P.; Prasad, S.; Atherton, J.; Parsonage, W.; Aldous, S.; Than, M.; Cullen, L. Chest pain workup in the presence of atrial fibrillation: Impacts on troponin testing, myocardial infarction diagnoses, and long-term prognosis. Eur Heart J. Acute Cardiovasc. Care 2022, 11, 772–781. [Google Scholar] [CrossRef]
- Goette, A.; Bukowska, A.; Lillig, C.H.; Lendeckel, U. Oxidative Stress and Microcirculatory Flow Abnormalities in the Ventricles during Atrial Fibrillation. Front. Physiol. 2012, 3, 236. [Google Scholar] [CrossRef] [Green Version]
- Costabel, J.P.; Burgos, L.M.; Trivi, M. The Significance Of Troponin Elevation In Atrial Fibrillation. J. Atr. Fibrillation 2017, 9, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevers, T.; Salvador, A.M.; Grodecki-Pena, A.; Knapp, A.; Velázquez, F.; Aronovitz, M.; Kapur, N.K.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ. Heart Fail. 2015, 8, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederichsen, S.Z.; Haugan, K.J.; Brandes, A.; Graff, C.; Krieger, D.; Kronborg, C.; Holst, A.G.; Nielsen, J.B.; Køber, L.; Højberg, S.; et al. Incidence and predictors of atrial fibrillation episodes as detected by implantable loop recorder in patients at risk: From the LOOP study. Am. Heart J. 2020, 219, 117–127. [Google Scholar] [CrossRef]
- Schäfer, S.; Zernecke, A. CD8(+) T Cells in Atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef]
- Tae Yu, H.; Youn, J.C.; Lee, J.; Park, S.; Chi, H.S.; Lee, J.; Choi, C.; Park, S.; Choi, D.; Ha, J.W.; et al. Characterization of CD8(+)CD57(+) T cells in patients with acute myocardial infarction. Cell. Mol. Immunol. 2015, 12, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, M.; Tabaraie, T.; Steffens, D.; Friebel, J.; Dörner, A.; Skurk, C.; Witkowski, M.; Stratmann, B.; Tschoepe, D.; Landmesser, U.; et al. MicroRNA-19a contributes to the epigenetic regulation of tissue factor in diabetes. Cardiovasc. Diabetol. 2018, 17, 34. [Google Scholar] [CrossRef]
- Witkowski, M.; Witkowski, M.; Saffarzadeh, M.; Friebel, J.; Tabaraie, T.; Ta Bao, L.; Chakraborty, A.; Dörner, A.; Stratmann, B.; Tschoepe, D.; et al. Vascular miR-181b controls tissue factor-dependent thrombogenicity and inflammation in type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Eckardt, L.; Valgimigli, M.; Lewalter, T.; Laeis, P.; Reimitz, P.-E.; Smolnik, R.; Zierhut, W.; Tijssen, J.G.; Vranckx, P. Clinical risk predictors in atrial fibrillation patients following successful coronary stenting: ENTRUST-AF PCI sub-analysis. Clin. Res. Cardiol. 2021, 110, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.Z.; Safford, M.M.; Muntner, P.; Khodneva, Y.; Dawood, F.Z.; Zakai, N.A.; Thacker, E.L.; Judd, S.; Howard, V.J.; Howard, G.; et al. Atrial Fibrillation and the Risk of Myocardial Infarction. JAMA Intern. Med. 2014, 174, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Borof, K.; Breithardt, G.; Camm, A.J.; Crijns, H.; Goette, A.; Kuck, K.H.; Metzner, A.; Vardas, P.; Vettorazzi, E.; et al. Early Rhythm Control in Patients With Atrial Fibrillation and High Comorbidity Burden. Circulation 2022, 146, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Magnussen, C.; Ozga, A.K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients With Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Jumeau, C.; Rupin, A.; Chieng-Yane, P.; Mougenot, N.; Zahr, N.; David-Dufilho, M.; Hatem, S.N. Direct Thrombin Inhibitors Prevent Left Atrial Remodeling Associated With Heart Failure in Rats. JACC Basic Transl. Sci. 2016, 1, 328–339. [Google Scholar] [CrossRef]
- Rao, S.V.; Kirsch, B.; Bhatt, D.L.; Budaj, A.; Coppolecchia, R.; Eikelboom, J.; James, S.K.; Jones, W.S.; Merkely, B.; Keller, L.; et al. A Multicenter, Phase 2, Randomized, Placebo-Controlled, Double-Blind, Parallel-Group, Dose-Finding Trial of the Oral Factor XIa Inhibitor Asundexian to Prevent Adverse Cardiovascular Outcomes Following Acute Myocardial Infarction. Circulation 2022, 146, 1196–1206. [Google Scholar] [CrossRef]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef]






| Control Group | Patients with First-Diagnosed AF | ||
|---|---|---|---|
| (n = 20) | (n = 80) | p-Value | |
| Male/Female | 10/10 (50%/50%) | 50/30 (62.5%/37.5%) | n.s. |
| CHA2DS2-VASc | 3.45 | 3.98 | n.s. |
| History of Heart Failure | 4/20 (20%) | 21/80 (26%) | n.s. |
| Ejection Fraction | 62% | 59% | n.s. |
| NT-pro BNP ng/L | 170.3 | 2153 | <0.0001 |
| Hypertension | 17/20 (85%) | 70/80 (87.5%) | n.s. |
| Age (years) | |||
| <65 | 9/29 (45%) | 22/80 (27.5%) | n.s. |
| 65–75 | 6/20 (30%) | 26/80 (32.5%) | n.s. |
| >75 | 5/20 (25%) | 32/80 (40%) | n.s. |
| Diabetes | 5/20 (25%) | 24/80 (30%) | n.s. |
| History of TIA/Stroke | 1/20 (5%) | 8/80 (10%) | n.s. |
| Body Weight (kg) | 82.95 | 85.93 | n.s. |
| BMI kg/m² | 27.97 | 27.55 | n.s. |
| PCT µg/L (ULN 0.09) | 0.07 | 0.07 | n.s. |
| CRP mg/L (ULN 5 mg/L) | 3.33 | 3.25 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friebel, J.; Witkowski, M.; Wegner, M.; Blöbaum, L.; Lammel, S.; Schencke, P.-A.; Jakobs, K.; Puccini, M.; Reißner, D.; Steffens, D.; et al. Cytotoxic CD8+ T Cells Are Involved in the Thrombo-Inflammatory Response during First-Diagnosed Atrial Fibrillation. Cells 2023, 12, 141. https://doi.org/10.3390/cells12010141
Friebel J, Witkowski M, Wegner M, Blöbaum L, Lammel S, Schencke P-A, Jakobs K, Puccini M, Reißner D, Steffens D, et al. Cytotoxic CD8+ T Cells Are Involved in the Thrombo-Inflammatory Response during First-Diagnosed Atrial Fibrillation. Cells. 2023; 12(1):141. https://doi.org/10.3390/cells12010141
Chicago/Turabian StyleFriebel, Julian, Marco Witkowski, Max Wegner, Leon Blöbaum, Stella Lammel, Philipp-Alexander Schencke, Kai Jakobs, Marianna Puccini, Daniela Reißner, Daniel Steffens, and et al. 2023. "Cytotoxic CD8+ T Cells Are Involved in the Thrombo-Inflammatory Response during First-Diagnosed Atrial Fibrillation" Cells 12, no. 1: 141. https://doi.org/10.3390/cells12010141