Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells
Abstract
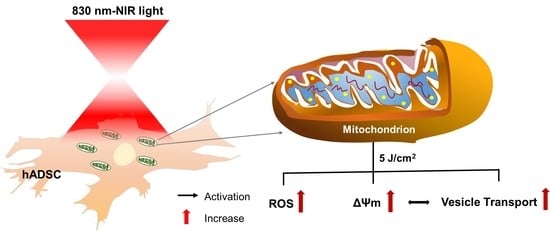
:1. Introduction
2. Materials and Methods
2.1. Experimental System
2.2. Cell Culture and Chemicals
2.3. Time-Lapse Rhodamine 123 Probe of Mitochondrial Membrane Potential in PBM
2.4. Detection of Mitochondrial Membrane Potential in PBM
2.5. Assessment of ROS in PBM
2.6. Vesicle Transport
2.7. Imaging Analysis
2.8. Statistical Analysis
3. Results
3.1. Time-Lapse Rh123 of Mitochondrial Membrane Potential in Photobiomodulation
3.2. Effects of PBM in Promoting the Mitochondrial Membrane Potential in hADSCs
3.3. Effects of PBM in Promoting ROS in hADSCs
3.4. Vesicle Transport in hADSCs after PBM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Guo, S.; Wang, Y.; Yu, Y. Adipose-derived stem cells and hyaluronic acid based gel compatibility, studied in vitro. Mol. Med. Rep. 2017, 16, 4095–4100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Ji, W.; Wang, L.; Chen, X.; Zhao, S.; Xu, Z.; Ge, R.; Guo, X. Differentiation of human adipose derived stem cells into Leydig-like cells with molecular compounds. J. Cell. Mol. Med. 2019, 23, 5956–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.-H.; Kuo, T.-L.; Kuo, K.-K.; Hsiao, C.-C. Human adipose-derived stem cells: Isolation, characterization and current application in regeneration medicine. Genom. Med. Biomark. Health Sci. 2011, 3, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Liu, H.-Y.; Chang, Y.-T.; Cheng, Y.-H.; Mersmann, H.J.; Kuo, W.-H.; Ding, S.-T. Isolation and differentiation of adipose-derived stem cells from porcine subcutaneous adipose tissues. J. Vis. Exp. 2016, 109, e53886. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Ji, B.; Wang, L.; Yan, Y.; Liu, Z.; Fang, S.; Wu, M.; Wang, Y.; Song, J. Human adipose-derived stem cells promote seawater-immersed wound healing by activating skin stem cells via the EGFR/MEK/ERK pathway. Stem Cells Int. 2019, 2019, 7135974. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Carpena, N.T.; Kang, B.J.; Lee, M.Y. Effects of Photobiomodulation on Stem Cells Important for Regenerative Medicine. Med. Lasers Eng. Basic Res. Clin. Appl. 2020, 9, 134–141. [Google Scholar] [CrossRef]
- Khan, I.; Rahman, S.U.; Tang, E.; Engel, K.; Hall, B.; Kulkarni, A.B.; Arany, P.R. Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-β1. Sci. Rep. 2021, 11, 13371. [Google Scholar] [CrossRef]
- Waterhouse, N.J.; Goldstein, J.C.; Von Ahsen, O.; Schuler, M.; Newmeyer, D.D.; Green, D.R. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol. 2001, 153, 319–328. [Google Scholar] [CrossRef]
- Engel, K.W.; Khan, I.; Arany, P.R. Cell lineage responses to photobiomodulation therapy. J. Biophotonics 2016, 9, 1148–1156. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.-Y.; Lee, M.Y.; Chung, P.-S.; Kim, S.; Choi, B.; Suh, M.-W.; Rhee, C.-K.; Jung, J.Y. Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Sci. Rep. 2019, 9, 19248. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. In Mitochondrial Bioenergetics, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 183–205. [Google Scholar]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Suski, J.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. In Mitochondrial Bioenergetics, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 357–381. [Google Scholar]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef]
- Xu, W.; Alizadeh, E.; Prasad, A. Force spectrum microscopy using mitochondrial fluctuations of control and atp-depleted cells. Biophys. J. 2018, 114, 2933–2944. [Google Scholar] [CrossRef] [Green Version]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Reyes, I.; Diebold, L.P.; Kong, H.; Schieber, M.; Huang, H.; Hensley, C.T.; Mehta, M.M.; Wang, T.; Santos, J.H.; Woychik, R. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 2016, 61, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Helmy, M.A.; Mohamed, A.F.; Rasheed, H.M.; Fayad, A.I. A protocol for primary isolation and culture of adipose-derived stem cells and their phenotypic profile. Alex. J. Med. 2020, 56, 42–50. [Google Scholar] [CrossRef]
- Wang, J.M.; Gu, Y.; Pan, C.J.; Yin, L.R. Isolation, culture and identification of human adipose-derived stem cells. Exp. Ther. Med. 2017, 13, 1039–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudifar, N.; Doran, P.M. Mesenchymal stem cells derived from human adipose tissue. In Cartilage Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 53–64. [Google Scholar]
- Wang, X.; Li, L.; Guan, R.; Zhu, D.; Song, N.; Shen, L. Emodin inhibits ATP-induced proliferation and migration by suppressing P2Y receptors in human lung adenocarcinoma cells. Cell. Physiol. Biochem. 2017, 44, 1337–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hameren, G.; Campbell, G.; Deck, M.; Berthelot, J.; Gautier, B.; Quintana, P.; Chrast, R.; Tricaud, N. In vivo real-time dynamics of ATP and ROS production in axonal mitochondria show decoupling in mouse models of peripheral neuropathies. Acta Neuropathol. Commun. 2019, 7, 86. [Google Scholar] [CrossRef]
- Imamura, H.; Nhat, K.P.H.; Togawa, H.; Saito, K.; Iino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. USA 2009, 106, 15651–15656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, H.A.; Khafagy, R.M. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat Sertoli cells: Possible role of mitochondrial fractions of Sertoli cells. Toxicol. Appl. Pharmacol. 2011, 252, 273–280. [Google Scholar] [CrossRef]
- Skjellegrind, H.K.; Fayzullin, A.; Johnsen, E.O.; Eide, L.; Langmoen, I.A.; Moe, M.C.; Vik-Mo, E.O. Short-term differentiation of glioblastoma stem cells induces hypoxia tolerance. Neurochem. Res. 2016, 41, 1545–1558. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, H.; Amini, A.; Fadaei Fathabady, F.; Mostafavinia, A.; Zare, F.; Ebrahimpour-Malekshah, R.; Ghalibaf, M.N.; Abrisham, M.; Rezaei, F.; Albright, R. Transplantation of photobiomodulation-preconditioned diabetic stem cells accelerates ischemic wound healing in diabetic rats. Stem Cell Res. Ther. 2020, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.P.; Copete, I.N.; Lopes, H.B.; Arany, P.R.; Marques, M.M.; Ferraz, E.P. Recovering the osteoblastic differentiation potential of mesenchymal stem cells derived from diabetic rats by photobiomodulation therapy. J. Biophotonics 2021, 14, e202000393. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.A.; do Valle, I.B.; Gleber-Netto, F.O.; Silva, T.A.; Oliveira, H.M.d.C.; de Oliveira, R.F.; Ferreira, L.d.A.Q.; Castilho, L.S.; Reis, P.H.; Prazeres, P.H. Nestin and NG2 transgenes reveal two populations of perivascular cells stimulated by photobiomodulation. J. Cell. Physiol. 2022. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Chen, A.C.-H.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response 2009, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose-Response 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Arany, P. Craniofacial wound healing with photobiomodulation therapy: New insights and current challenges. J. Dent. Res. 2016, 95, 977–984. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Moon, J.H.; Jung, J.Y.; Oh, C.; Ahn, J.-C.; Chung, P.-S. Effect of photobiomodulation therapy on neuronal injuries by ouabain: The regulation of Na, K-ATPase; Src; and mitogen-activated protein kinase signaling pathway. BMC Neurosci. 2019, 20, 19. [Google Scholar] [CrossRef]
- Mochizuki-Oda, N.; Kataoka, Y.; Cui, Y.; Yamada, H.; Heya, M.; Awazu, K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci. Lett. 2002, 323, 207–210. [Google Scholar] [CrossRef]
- Groeger, G.; Quiney, C.; Cotter, T.G. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Signal. 2009, 11, 2655–2671. [Google Scholar] [CrossRef]
- Huang, L.; Wu, S.; Xing, D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3β signaling pathway. J. Cell. Physiol. 2011, 226, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, R.; Silva, N.d.; Zangaro, R.; Pacheco, M.; Da Matta, R.; Pacheco-Soares, C. Mitochondrial membrane potential after low-power laser irradiation. Lasers Med. Sci. 2004, 18, 204–206. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Elustondo, P.; Nichols, M.; Negoda, A.; Thirumaran, A.; Zakharian, E.; Robertson, G.; Pavlov, E. Mitochondrial permeability transition pore induction is linked to formation of the complex of ATPase C-subunit, polyhydroxybutyrate and inorganic polyphosphate. Cell Death Discov. 2016, 2, 16070. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341, 233–249. [Google Scholar] [CrossRef]
- Brookes, P.S. Mitochondrial H+ leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, S.; Brand, M. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 2003, 31, 1300–1301. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xing, D.; Liu, L.; Tang, Y. Microtubule-dependent trafficking of h-ras to cell surface after low-power laser irradiation. In Advances in Biomedical Photonics and Imaging; World Scientific: Singapore, 2008; pp. 150–155. [Google Scholar]
- Cooper, G.M. The Cell, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem. Photobiol. 1997, 66, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Sarti, P.; Forte, E.; Mastronicola, D.; Giuffrè, A.; Arese, M. Cytochrome c oxidase and nitric oxide in action: Molecular mechanisms and pathophysiological implications. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 610–619. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.-C.; Hang, N.-L.-T.; Colley, M.M.S.; Chang, J.; Hsiao, Y.-C.; Lu, L.-S.; Li, B.-S.; Chang, C.-J.; Yang, T.-S. Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells. Cells 2022, 11, 972. https://doi.org/10.3390/cells11060972
Pan L-C, Hang N-L-T, Colley MMS, Chang J, Hsiao Y-C, Lu L-S, Li B-S, Chang C-J, Yang T-S. Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells. Cells. 2022; 11(6):972. https://doi.org/10.3390/cells11060972
Chicago/Turabian StylePan, Li-Chern, Nguyen-Le-Thanh Hang, Mamadi M.S Colley, Jungshan Chang, Yu-Cheng Hsiao, Long-Sheng Lu, Bing-Sian Li, Cheng-Jen Chang, and Tzu-Sen Yang. 2022. "Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells" Cells 11, no. 6: 972. https://doi.org/10.3390/cells11060972