Effects of Simvastatin on Cartilage Homeostasis in Steroid-Induced Osteonecrosis of Femoral Head by Inhibiting Glucocorticoid Receptor
Abstract
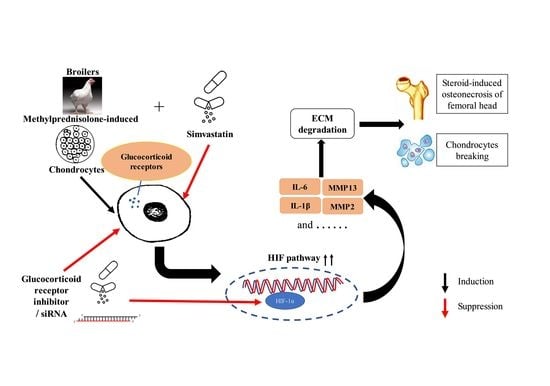
:1. Introduction
2. Materials and Methods
2.1. Simvastatin Treatment
2.2. Animals and Treatments
2.2.1. Animal Treatment and Sample Collection
2.2.2. Histopathology
2.2.3. Bone Biomechanical Tests
2.2.4. Chicken Serum and Plasma Assays
2.2.5. RNA Extraction and Real-Time Quantitative PCR
2.2.6. Western Blotting Analysis
2.3. Cells and Treatments
2.3.1. Primary Chondrocytes Isolation, Culture, and Identification
2.3.2. Staining of Cell
2.3.3. Cell Viability Assay and Treatment
2.3.4. Apoptosis Detection
2.3.5. ELISA Assay
2.3.6. Immunocytofluorescence Analysis
2.3.7. GR Inhibitor Treatment
2.3.8. Small Interfering RNA
2.4. Statistical Analysis
3. Results
3.1. Experiment Results
3.1.1. SMV Could Lower the Incidence of SONFH
3.1.2. MP Did Not Affect Lipid Metabolism
3.1.3. SMV Mitigated the Imbalance of Homeostasis Induced by MP via Inhibiting HIF Pathway
3.1.4. SMV Mitigated the ECM Degradation and Apoptosis in MP-Treated Chondrocytes
3.1.5. SMV Inhibited the HIF Pathway to Restore Cartilage Homeostasis In Vitro
3.1.6. SMV Restrained the Expression of GR
3.1.7. GR Expression Effected the Level of HIF Pathway and Homeostasis in Chondrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yao, X.; Yu, S.; Jing, X.; Guo, J.; Sun, K.; Guo, F.; Sun, K.; Guo, F.; Ye, Y. PTEN inhibitor VO-OHpic attenuates GC-associated endothelial progenitor cell dysfunction and osteonecrosis of the femoral head via activating Nrf2 signaling and inhibiting mitochondrial apoptosis pathway. Stem Cell Res. Ther. 2020, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Marston, S.B.; Gillingham, K.; Bailey, R.F.; Cheng, E.Y. Osteonecrosis of the femoral head after solid organ transplantation—A prospective study. J. Bone Jt. Surg. Am. Vol. 2002, 84a, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Hogan, E.A.; Borrelli, M.J.; Liachenko, S.; O’Brien, C.A.; Manolagas, S.C. The Pathophysiological Sequence of Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Male Mice. Endocrinology 2017, 158, 3817–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.M.; Liu, B.Y.; Wang, B.J.; Zhao, D.W. Early diagnosis and treatment of steroid-induced osteonecrosis of the femoral head. Int. Orthop. 2019, 43, 1083–1087. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.G.; Zhang, J.Y.; Zhuo, Y.G.; Hong, X.; Ye, J.; Tang, S.S.; Liu, N.; Zhang, Y. ARG2, MAP4K5 and TSTA3 as Diagnostic Markers of Steroid-Induced Osteonecrosis of the Femoral Head and Their Correlation with Immune Infiltration. Front. Genet. 2021, 12, 691465. [Google Scholar] [CrossRef]
- Mont, M.A.; Jones, L.C.; Hungerford, D.S. Nontraumatic osteonecrosis of the femoral head, ten years later. J. Bone Jt. Surg. Am. 2006, 88, 1117–1132. [Google Scholar]
- Mont, M.A.; Hungerford, D.S. Non-traumatic avascular necrosis of the femoral head. J. Bone Jt. Surg. Am. 1995, 77, 459–474. [Google Scholar] [CrossRef]
- Yue, J.; Yu, H.; Liu, P.; Wen, P.; Zhang, H.; Guo, W.; Zhang, Q. Preliminary study of icariin indicating prevention of steroid-induced osteonecrosis of femoral head by regulating abnormal expression of miRNA-335 and protecting the functions of bone microvascular endothelial cells in rats. Gene 2021, 766, 145128. [Google Scholar] [CrossRef]
- Huang, C.; Wen, Z.; Niu, J.; Lin, S.; Wang, W. Steroid-Induced Osteonecrosis of the Femoral Head: Novel Insight Into the Roles of Bone Endothelial Cells in Pathogenesis and Treatment. Front. Cell Dev. Biol. 2021, 9, 777697. [Google Scholar] [CrossRef]
- Packialakshmi, B.; Liyanage, R.; Lay, J.J.; Okimoto, R.; Rath, N. Prednisolone-induced predisposition to femoral head separation and the accompanying plasma protein changes in chickens. Biomark Insights 2015, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, S.; Zhou, Z. Cartilage Homeostasis Affects Femoral Head Necrosis Induced by Methylprednisolone in Broilers. Int. J. Mol. Sci. 2020, 21, 4841. [Google Scholar] [CrossRef] [PubMed]
- Pfander, D.; Swoboda, B.; Cramer, T. The role of HIF-1alpha in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudoh, K.; Nakamura, H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: Involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2005, 7, R904–R914. [Google Scholar] [CrossRef] [Green Version]
- Stegen, S.; Laperre, K.; Eelen, G.; Rinaldi, G.; Fraisl, P.; Torrekens, S.; van Looveren, R.; Loopmans, S.; Bultynck, G.; Vinckier, S.; et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature 2019, 565, 511–515. [Google Scholar] [CrossRef]
- Becker, P.B.; Gloss, B.; Schmid, W.; Strahle, U.; Schutz, G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature 1986, 324, 686–688. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Meng, D.; Zhang, X.; Yuan, D. Effect of psoralen on the expression of PPARgamma, osteocalcin, and trabecular bone area in rabbits with steroid-induced avascular necrosis of the femoral head. J. Orthop. Surg. Res. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yao, L.; Wang, B.; Liu, Z.; Ma, K. Tao-Hong-Si-Wu Decoction ameliorates steroid-induced avascular necrosis of the femoral head by regulating the HIF-1alpha pathway and cell apoptosis. Biosci. Trends. 2016, 10, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Leung, B.P.; Sattar, N.; Crilly, A.; Prach, M.; McCarey, D.W.; Payne, H.; Madhok, R.; Campbell, C.; Gracie, J.A.; Liew, F.W.; et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J. Immunol. 2003, 170, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Ruan, F.; Zheng, Q.; Wang, J. Mechanisms of bone anabolism regulated by statins. Biosci. Rep. 2012, 32, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Palmer, G.; Chobaz, V.; Talabot-Ayer, D.; Taylor, S.; So, A.; Gabay, C.; Busso, N. Assessment of the efficacy of different statins in murine collagen-induced arthritis. Arthritis Rheum. 2004, 50, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tonkin, A.; Jones, G.; Hill, C.; Ding, C.; Wluka, A.E.; Forbes, A.; Cicuttini, F.M. Does statin use have a disease modifying effect in symptomatic knee osteoarthritis? Study protocol for a randomised controlled trial. Trials 2015, 16, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudoh, K.; Karasawa, R. Statin prevents chondrocyte aging and degeneration of articular cartilage in osteoarthritis (OA). Aging 2010, 2, 990–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, E.A.; Novaes, R.D.; Nakagaki, W.R.; Fernandes, G.J.; Garcia, J.A.; Camilli, J.A. Metabolic and structural bone disturbances induced by hyperlipidic diet in mice treated with simvastatin. Int. J. Exp. Pathol. 2015, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Spector, T.D. Statins as modulators of bone formation. Arthritis Res. 2002, 4, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Matsuda, S.; Nakayama, K.; Fukagawa, S.; Miura, H.; Iwamoto, Y. Mevastatin reduces cartilage degradation in rabbit experimental osteoarthritis through inhibition of synovial inflammation. Osteoarthr. Cartilage. 2009, 17, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Funk, J.L.; Chen, J.; Downey, K.J.; Clark, R.A. Bone protective effect of simvastatin in experimental arthritis. J. Rheumatol. 2008, 35, 1083–1091. [Google Scholar]
- Jadhav, S.B.; Jain, G.K. Statins and osteoporosis: New role for old drugs. J. Pharm. Pharmacol. 2006, 58, 3–18. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Selvi, E.; Lorenzini, S.; Bisogno, S.; Baldari, C.T.; Galeazzi, M.; Leigh-Pasini, F. Statins and the joint: Multiple targets for a global protection? Semin. Arthritis Rheum. 2011, 40, 430–446. [Google Scholar] [CrossRef]
- Okazaki, S.; Nishitani, Y.; Nagoya, S.; Kaya, M.; Yamashita, T.; Matsumoto, H. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology 2009, 48, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.-M.; Audigie, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Erken, H.Y.; Ofluoglu, O.; Aktas, M.; Topal, C.; Yildiz, M. Effect of pentoxifylline on histopathological changes in steroid-induced osteonecrosis of femoral head: Experimental study in chicken. Int. Orthop. 2012, 36, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Reddi, A.H. Cartilage morphogenetic proteins: Role in joint development, homoeostasis, and regeneration. Ann. Rheum. Dis. 2003, 62 (Suppl. S2), ii73–ii78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.; Paschos, N.K.; Hu, J.C.; Athanasiou, K. Articular cartilage tissue engineering: The role of signaling molecules. Cell Mol. Life Sci. 2016, 73, 1173–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686. [Google Scholar] [CrossRef]
- Sophia, F.A.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Vettori, A.; Greenald, D.; Wilson, G.K.; Peron, M.; Facchinello, N.; Markham, E.; Sinnakaruppan, M.; Matthews, L.C.; McKeating, J.A.; Argenton, F.; et al. Glucocorticoids promote Von Hippel Lindau degradation and Hif-1alpha stabilization. Proc. Natl. Acad. Sci. USA 2017, 114, 9948–9953. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Wang, G.J.; Su, C.C.; Balian, G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin. Orthop. Relat. Res. 1997, 344, 8–19. [Google Scholar] [CrossRef]
- Wang, G.J.; Cui, Q.; Balian, G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin. Orthop. Relat. Res. 2000, 370, 295–310. [Google Scholar] [CrossRef]
- Cook, M.E. Skeletal deformities and their causes: Introduction. Poult. Sci. 2000, 79, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Toney, C.B.; Seamon, J.; Cui, Q. Blood supply to the chicken femoral head. Comp. Med. 2010, 60, 295–299. [Google Scholar] [PubMed]
- Matzelle, M.M.; Shaw, A.T.; Baum, R.; Maeda, Y.; Li, J.; Karmakar, S.; Manning, C.A.; Walsh, N.C.; Rosen, V.; Gravallese, E.M. Inflammation in arthritis induces expression of BMP3, an inhibitor of bone formation. Scand. J. Rheumatol. 2016, 45, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, J.; Morse, M.; Levin, V. Effect of simvastatin on bone markers in osteopenic women, a placebo-controlled, dose-ranging trial. BMC Musculoskelet. Disord. 2002, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Tokudome, S.; Sano, M.; Shinmura, K.; Matsuhashi, T.; Morizane, S.; Moriyama, H.; Tamaki, K.; Hayashida, K.; Nakanishi, H.; Yoshikawa, N.; et al. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J. Clin. Investig. 2009, 119, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Dardzinski, B.J.; Smith, S.L.; Towfighi, J.; Williams, G.D.; Vannucci, R.C.; Smith, M.B. Increased plasma beta-hydroxybutyrate, preserved cerebral energy metabolism, and amelioration of brain damage during neonatal hypoxia ischemia with dexamethasone pretreatment. Pediatr. Res. 2000, 48, 248–255. [Google Scholar] [CrossRef] [Green Version]
- FaFacchinello, N.; Skobo, T.; Meneghetti, G.; Colletti, E.; Dinarello, A.; Tiso, N.; Costa, R.; Gioacchini, G.; Carnevali, O.; Argenton, F.; et al. nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Sci. Rep. 2017, 7, 4371. [Google Scholar] [CrossRef] [Green Version]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Model Mech. 2019, 12, dmm037887. [Google Scholar]
- Marchi, D.; van Eeden, F.J. Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic. Cells 2021, 10, 3441. [Google Scholar] [CrossRef]
- Arnaud, C.; Braunersreuther, V.; Mach, F. Toward Immunomodulatory and Anti-Inflammatory Properties of Statins. Trends Cardiovasc. Med. 2005, 15, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A. Vascular changes after interference with the blood flow of the femoral head of the rabbit. J. Bone Jt. Surg. Br. 1957, 39-B, 763–777. [Google Scholar] [CrossRef] [PubMed]













| Group (n = 6) | FHN Evaluation Score | Total Morbidity (%) | Total Score | Amount | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | |||||
| 42 Day | Group C | 9 | 2 | 1 | 25.00 | 4 | 12 |
| Group M | 5 | 4 | 3 | 58.33 | 10 | 12 | |
| Group MS | 8 | 2 | 2 | 33.33 | 6 | 12 | |
| Group S | 10 | 2 | 0 | 16.67 | 2 | 12 | |
| 56 Day | Group C | 10 | 2 | 0 | 16.67 | 2 | 12 |
| Group M | 6 | 3 | 3 | 50.00 | 9 | 12 | |
| Group MS | 8 | 3 | 1 | 33.33 | 5 | 12 | |
| Group S | 9 | 3 | 0 | 25.00 | 3 | 12 | |
| ICRS Score | Group C (n = 6) | Group M (n = 6) | Group MS (n = 6) | Group S (n = 6) |
|---|---|---|---|---|
| Grade 0 | 4 | 1 | 3 | 3 |
| Grade 1 | 6 | 3 | 4 | 7 |
| Grade 2 | 2 | 4 | 4 | 1 |
| Grade 3 | 0 | 1 | 1 | 1 |
| Grade 4 | 0 | 3 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Lin, L.; Liu, K.; Jiang, Y.; Zhou, Z. Effects of Simvastatin on Cartilage Homeostasis in Steroid-Induced Osteonecrosis of Femoral Head by Inhibiting Glucocorticoid Receptor. Cells 2022, 11, 3945. https://doi.org/10.3390/cells11243945
Yu Y, Lin L, Liu K, Jiang Y, Zhou Z. Effects of Simvastatin on Cartilage Homeostasis in Steroid-Induced Osteonecrosis of Femoral Head by Inhibiting Glucocorticoid Receptor. Cells. 2022; 11(24):3945. https://doi.org/10.3390/cells11243945
Chicago/Turabian StyleYu, Yaling, Lishan Lin, Kangping Liu, Yixin Jiang, and Zhenlei Zhou. 2022. "Effects of Simvastatin on Cartilage Homeostasis in Steroid-Induced Osteonecrosis of Femoral Head by Inhibiting Glucocorticoid Receptor" Cells 11, no. 24: 3945. https://doi.org/10.3390/cells11243945