A Spontaneous Inversion of the X Chromosome Heterochromatin Provides a Tool for Studying the Structure and Activity of the Nucleolus in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flies
2.2. Morphological Analyses and Microscopy of Polytene Chromosomes
2.3. MinIon Sequencing
2.4. Nanopore Sequencing Data Treatment and Breakpoint Detection
2.5. PCR Primers and FISH Probes
- P1 5′-ACTTTGATGCCTGCTCCAGT-3′;
- P2 5′-ATTGACACAACCCATTTAAGAG-3′;
- P3 5′-GGGCAGGTTCGAGGTTGGGAAGC-3′;
- P4 5′-CCTTTGCCAGTTGAGTTTTCTATGCCG-3′;
- P5 5′-GCCATTGTCCAGCAATCGCCAAA-3′;
- P6 5′-GCCAAGTACTTTGCCATCTTTCG-3′;
- P7 5′-GTCTGGAGCGAGAGCGGCCCTC-3′;
- P8 5′-CGCGCACGCTTTCTGCAAAA-3′;
- P9 5′-CGCTTAAGAGCGTAAAATGCATGGAG-3′.
2.6. Fluorescence In Situ Hybridization (FISH) and Indirect Immunostaining
2.7. EU Incorporation and Detection
2.8. Data Availability
3. Results
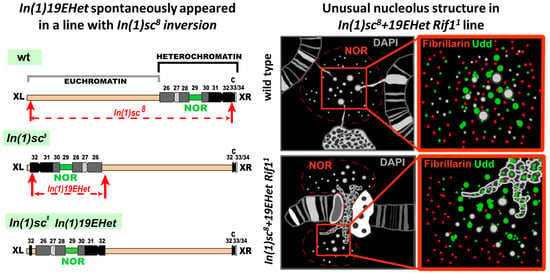
3.1. Detection of a New Inversion That Spontaneously Appeared in BDSC Line #798 with In(1)sc8 Inversion
3.2. Detection of Inversion Breakpoints Using Nanopore Sequencing
3.3. Morphology of the Nucleolar Organizer in In(1)sc8 + 19EHet; Rif11 Polytene Chromosomes
3.4. Identification of the Transcriptionally Active Part of the Nucleolus in In(1)sc8 + 19EHet; Rif11 Polytene Chromosomes
3.5. The In(1)sc8 + 19EHet; Rif11 Genotype Allows Visualization of the Y Chromosome Nucleolus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoskins, R.A.; Smith, C.D.; Carlson, J.W.; Carvalho, A.B.; Halpern, A.; Kaminker, J.S.; Kennedy, C.; Mungall, C.J.; Sullivan, B.A.; Sutton, G.G.; et al. Heterochromatic sequences in a Drosophila whole-genome shotgun assembly. Genome Biol. 2002, 3, RESEARCH0085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskins, R.A.; Carlson, J.W.; Wan, K.H.; Park, S.; Mendez, I.; Galle, S.E.; Booth, B.W.; Pfeiffer, B.D.; George, R.A.; Svirskas, R.; et al. The release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 2015, 25, 445–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritossa, F.M.; Spiegelman, S. Localization of DNA complementary to ribosomal RNA in the nucleolus organiser region of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1965, 53, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Hilliker, A.J.; Appels, R. Pleiotropic effects associated with the deletion of heterochromatin surrounding rDNA on the X chromosome of Drosophila. Chromosoma 1982, 86, 469–490. [Google Scholar] [CrossRef]
- Kolesnikova, T.D.; Koriakov, D.E.; Semeshin, V.F.; Beliaeva, E.S.; Zhimulev, I.F. Interline differences in morphology of the precentromeric region of polytene X-chromosome in Drosophila melanogaster salivary glands. Genetika 2001, 37, 1632–1641. [Google Scholar] [PubMed]
- Parry, D.M.; Sandler, L. The Genetic Identification of a heterochromatic segment on the X chromosome of Drosophila melanogaster. Genetics 1974, 77, 535–539. [Google Scholar] [CrossRef]
- Gatti, M.; Pimpinelli, S. Functional elements in Drosophila melanogaster heterochromatin. Annu. Rev. Genet. 1992, 26, 239–275. [Google Scholar] [CrossRef]
- Wellauer, P.K.; Dawid, I.B.; Tartof, K.D. X and Y chromosomal ribosomal DNA of Drosophila: Comparison of spacers and insertions. Cell 1978, 14, 269–278. [Google Scholar] [CrossRef]
- Tautz, D.; Hancock, J.M.; Webb, D.A.; Tautz, C.; Dover, G.A. Complete sequences of the rRNA genes of Drosophila melanogaster. Mol. Biol. Evol. 1988, 5, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Lyckegaard, E.M.; Clark, A.G. Evolution of ribosomal RNA gene copy number on the sex chromosomes of Drosophila melanog aster. Mol. Biol. Evol. 1991, 8, 458–474. [Google Scholar] [CrossRef]
- Bianciardi, A.; Boschi, M.; Swanson, E.E.; Belloni, M.; Robbins, L.G. Ribosomal DNA organization before and after magnification in Drosophila melanogaster. Genetics 2012, 191, 703–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyckegaard, E.M.; Clark, A.G. Ribosomal DNA and Stellate gene copy number variation on the Y chromosome of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1989, 86, 1944–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartof, K.D. Regulation of ribosomal RNA gene multiplicity in Drosophila melanogaster. Genetics 1973, 73, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Terracol, R.; Prud’homme, N. Differential elimination of rDNA genes in bobbed mutants of Drosophila melanogaster. Mol. Cell Biol. 1986, 6, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Mohan, J.; Ritossa, F.M. Regulation of ribosomal RNA synthesis and its bearing on the bobbed phenotype in Drosophila melanogaster. Dev. Biol. 1970, 22, 495–512. [Google Scholar] [CrossRef]
- Lu, K.L.; Nelson, J.O.; Watase, G.J.; Warsinger-Pepe, N.; Yamashita, Y.M. Transgenerational dynamics of rDNA copy number in Drosophila male germline stem cells. Elife 2018, 7, e32421. [Google Scholar] [CrossRef]
- Iarovaia, O.V.; Minina, E.P.; Sheval, E.V.; Onichtchouk, D.; Dokudovskaya, S.; Razin, S.V.; Vassetzky, Y.S. Nucleolus: A central hub for nuclear functions. Trends Cell Biol. 2019, 29, 647–659. [Google Scholar] [CrossRef]
- Pirogov, S.A.; Gvozdev, V.A.; Klenov, M.S. Long noncoding RNAs and stress response in the nucleolus. Cells 2019, 8, 668. [Google Scholar] [CrossRef] [Green Version]
- Greil, F.; Ahmad, K. Nucleolar dominance of the Y chromosome in Drosophila melanogaster. Genetics 2012, 191, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Sackton, T.B.; Martinsen, L.; Lemos, B.; Eickbush, T.H.; Hartl, D.L. Y chromosome mediates ribosomal DNA silencing and modulates the chromatin state in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 9941–9946. [Google Scholar] [CrossRef]
- Warsinger-Pepe, N.; Li, D.; Yamashita, Y.M. Regulation of nucleolar dominance in Drosophila melanogaster. Genetics 2020, 214, 991–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotov, A.A.; Bazylev, S.S.; Adashev, V.E.; Shatskikh, A.S.; Olenina, L.V. Drosophila as a model system for studying of the evolution and functional specialization of the Y chromosome. Int. J. Mol. Sci. 2022, 23, 4184. [Google Scholar] [CrossRef] [PubMed]
- Beadle, G.W.; Sturtevant, A.H. X Chromosome inversions and meiosis in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1935, 21, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, M.J.; Pagie, L.; Talhout, W.; Nieuwland, M.; Kerkhoven, R.M.; van Steensel, B. High-resolution mapping of heterochromatin redistribution in a Drosophila position-effect variegation model. Epigenetics Chromatin 2009, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidorov, B. A Study of step-allelomorphism in Drosophila melanogaster. A case of origination of an allelomorph of Scute producing simultaneously characters of ’Hairy Wing’ (Mutation Scute-8). Zhurnal Eksperimental Noi Biol. I Meditsiny 7 1931, 7, 28–40. [Google Scholar]
- Gershenson, S. Studies on the genetically inert region of the X-chromosome of Drosophila. I. Behaviour of an X-chromosome deficient for a part of its inert region. J. Genet. 1933, 28, 297–313. [Google Scholar] [CrossRef]
- Sturtevant, A.H.; Beadle, G.W. The relations of inversions in the X chromosome of Drosophila melanogaster to crossing over and disjunction. Genetics 1936, 21, 554–604. [Google Scholar] [CrossRef]
- Miller, D.E.; Cook, K.R.; Yeganeh Kazemi, N.; Smith, C.B.; Cockrell, A.J.; Hawley, R.S.; Bergman, C.M. Rare recombination events generate sequence diversity among balancer chromosomes in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2016, 113, E1352–E1361. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.B. The Phenomenon of position effect. Adv. Genet. 1950, 3, 73–115. [Google Scholar] [CrossRef]
- Hannah, A. Localization and function of heterochromatin in Drosophila melanogaster. Adv. Genet. 1951, 4, 87–125. [Google Scholar] [CrossRef]
- Baker, W.K. Position-effect variegation. Adv. Genet. 1968, 14, 133–169. [Google Scholar] [PubMed]
- Spofford, J. Position-effect variegation in Drosophila. In The Genetics and Biology of Drosophila; Ashburner, M., Novitski, E., Eds.; Academic Press: New York, NY, USA, 1976; Volume 1, pp. 955–1018. [Google Scholar]
- Appels, R.; Hilliker, A.J. The cytogenetic boundaries of the rDNA region within heterochromatin in the X chromosome of Drosophila melanogaster and their relation to male meiotic pairing sites. Genet. Res. 1982, 39, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.E.; Cook, K.R.; Hawley, R.S. The joy of balancers. PLoS Genet. 2019, 15, e1008421. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Ganesamoorthy, D.; Duarte, T.; Cao, M.D.; Hoggart, C.J.; Coin, L.J.M. NpInv: Accurate detection and genotyping of inversions using long read sub-alignment. BMC Bioinform. 2018, 19, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solodovnikov, A.; Lavrov, S. Exact breakpoints of the In(1)wm4 rearrangement. MicroPubl. Biol. 2022. [Google Scholar] [CrossRef]
- Munden, A.; Rong, Z.; Sun, A.; Gangula, R.; Mallal, S.; Nordman, J.T. Rif1 inhibits replication fork progression and controls DNA copy number in Drosophila. Elife 2018, 7, e39140. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, T.D.; Kolodyazhnaya, A.V.; Pokholkova, G.V.; Schubert, V.; Dovgan, V.V.; Romanenko, S.A.; Prokopov, D.Y.; Zhimulev, I.F. Effects of mutations in the Drosophila melanogaster Rif1 gene on the replication and underreplication of pericentromeric heterochromatin in salivary gland polytene chromosomes. Cells 2020, 9, 1501. [Google Scholar] [CrossRef]
- Zhimulev, I.F.; Vlassova, I.E.; Belyaeva, E.S. Cytogenetic analysis of the 2B3-4--2B11 region of the X chromosome of Drosophila melanogaster. III. Puffing disturbance in salivary gland chromosomes of homozygotes for Mutation l(1)Pp1t10. Chromosoma 1982, 85, 659–672. [Google Scholar] [CrossRef]
- Weisshart, K.D.; Fuchs, J.; Schubert, V. Structured illumination microscopy (SIM) and photoactivated localization microscopy (PALM) to analyze the abundance and distribution of RNA Polymerase II molecules on flow-sorted Arabidopsis nuclei. Bio-Protoc. 2016, 6, e1725. [Google Scholar] [CrossRef] [Green Version]
- Koetsier, P.A.G.; Cantor, E.J. A simple approach for effective shearing and reliable concentration measurement of ultra-high-molecular-weight DNA. Biotechniques 2021, 71, 439–444. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blankenberg, D.; Gordon, A.; Von Kuster, G.; Coraor, N.; Taylor, J.; Nekrutenko, A.; the Galaxy Team. Manipulation of FASTQ data with Galaxy. Bioinformatics 2010, 26, 1783–1785. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Ekhteraei-Tousi, S.; Lewerentz, J.; Larsson, J. The X-linked 1.688 satellite in Drosophila melanogaster promotes specific targeting by Painting of Fourth. Genetics 2018, 208, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tautz, D.; Dover, G.A. Transcription of the tandem array of ribosomal DNA in Drosophila melanogaster does not terminate at any fixed point. EMBO J. 1986, 5, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shalaby, N.A.; Buszczak, M. Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science 2014, 343, 298–301. [Google Scholar] [CrossRef] [Green Version]
- Belyaeva, E.S.; Goncharov, F.P.; Demakova, O.V.; Kolesnikova, T.D.; Boldyreva, L.V.; Semeshin, V.F.; Zhimulev, I.F. Late replication domains in polytene and non-polytene cells of Drosophila melanogaster. PLoS ONE 2012, 7, e30035. [Google Scholar] [CrossRef] [Green Version]
- Belyaeva, E.S.; Zhimulev, I.F.; Volkova, E.I.; Alekseyenko, A.A.; Moshkin, Y.M.; Koryakov, D.E. Su(UR)ES: A gene suppressing DNA underreplication in intercalary and pericentric heterochromatin of Drosophila melanogaster polytene chromosomes. Proc. Natl. Acad. Sci. USA 1998, 95, 7532–7537. [Google Scholar] [CrossRef] [Green Version]
- Long, E.O.; Dawid, I.B. Expression of ribosomal DNA insertions in Drosophila melanogaster. Cell 1979, 18, 1185–1196. [Google Scholar] [CrossRef]
- Ye, J.; Eickbush, T.H. Chromatin structure and transcription of the R1- and R2-inserted rRNA genes of Drosophila melanogaster. Mol. Cell Biol. 2006, 26, 8781–8790. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Fefelova, E.; Ninova, M.; Chen, Y.-C.A.; Aravin, A.A. Repression of interrupted and intact rDNA by the SUMO pathway in Drosophila melanogaster. Elife 2020, 9, e52416. [Google Scholar] [CrossRef]
- Fefelova, E.A.; Pleshakova, I.M.; Mikhaleva, E.A.; Pirogov, S.A.; Poltorachenko, V.A.; Abramov, Y.A.; Romashin, D.D.; Shatskikh, A.S.; Blokh, R.S.; Gvozdev, V.A.; et al. Impaired function of rDNA transcription initiation machinery leads to derepression of ribosomal genes with insertions of R2 retrotransposon. Nucleic Acids Res. 2022, 50, 867–884. [Google Scholar] [CrossRef] [PubMed]
- Plata, M.P.; Kang, H.J.; Zhang, S.; Kuruganti, S.; Hsu, S.-J.; Labrador, M. Changes in chromatin structure correlate with transcriptional activity of nucleolar rDNA in polytene chromosomes. Chromosoma 2009, 118, 303–322. [Google Scholar] [CrossRef]
- Endow, S.A. Nucleolar dominance in polytene cells of Drosophila. Proc. Natl. Acad. Sci. USA 1983, 80, 4427–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotta, G.; Ebert, A.; Krauss, V.; Fischer, A.; Hoffmann, J.; Rea, S.; Jenuwein, T.; Dorn, R.; Reuter, G. Central role of Drosophila SU(VAR)3–9 in Histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 2002, 21, 1121–1131. [Google Scholar] [CrossRef]
- Schotta, G.; Ebert, A.; Reuter, G. SU(VAR)3-9 is a conserved key function in heterochromatic gene silencing. Genetica 2003, 117, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Elgin, S.C.R.; Reuter, G. Position-Effect Variegation, Heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 2013, 5, a017780. [Google Scholar] [CrossRef] [Green Version]
- Demakova, O.V.; Pokholkova, G.V.; Kolesnikova, T.D.; Demakov, S.A.; Andreyeva, E.N.; Belyaeva, E.S.; Zhimulev, I.F. The SU(VAR)3-9/HP1 complex differentially regulates the compaction state and degree of underreplication of X chromosome pericentric heterochromatin in Drosophila melanogaster. Genetics 2007, 175, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Andreyeva, E.N.; Kolesnikova, T.D.; Demakova, O.V.; Mendez-Lago, M.; Pokholkova, G.V.; Belyaeva, E.S.; Rossi, F.; Dimitri, P.; Villasante, A.; Zhimulev, I.F. High-resolution analysis of Drosophila heterochromatin organization Using SuUR Su(Var)3-9 double mutants. Proc. Natl. Acad. Sci. USA 2007, 104, 12819–12824. [Google Scholar] [CrossRef] [Green Version]
- Jacobs-Lorena, M. Dosage of 5S and ribosomal genes during oogenesis of Drosophila melanogaster. Dev. Biol. 1980, 80, 134–145. [Google Scholar] [CrossRef]
- Spear, B.B.; Gall, J.G. Independent control of ribosomal gene replication in polytene chromosomes of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1973, 70, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Eickbush, D.G.; Ye, J.; Zhang, X.; Burke, W.D.; Eickbush, T.H. Epigenetic regulation of retrotransposons within the nucleolus of Drosophila. Mol. Cell Biol. 2008, 28, 6452–6461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Eickbush, T.H. The pattern of R2 retrotransposon activity in natural populations of Drosophila simulans reflects the dynamic nature of the rDNA Locus. PLoS Genet. 2009, 5, e1000386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preuss, S.; Pikaard, C.S. rRNA gene silencing and nucleolar dominance: Insights into a chromosome-scale epigenetic on/off switch. Biochim. Biophys. Acta 2007, 1769, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, S.; Vitins, A.; Pikaard, C.S. Nucleolar dominance and ribosomal RNA gene silencing. Curr Opin. Cell Biol. 2010, 22, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, R.S.; Marcus, C.H. Recombinational controls of rDNA redundancy in Drosophila. Annu. Rev. Genet. 1989, 23, 87–120. [Google Scholar] [CrossRef] [PubMed]








| Stock | Origin | Genotype | Comments | Source |
|---|---|---|---|---|
| Rif11 | Deletion obtained via CRISPR-based mutagenesis; frameshift mutations at amino acid position 14. No detectable Rif1 protein. FBal0343570 | w1118; Rif11 | Used as a source of the Rif11 mutation | Kindly provided by Jared Nordman [37] |
| #798 “main copy” (BDSC) | Sub-lines created in 2012 in BDSC by splitting line #798 | In(1)sc8, In(1)19EHet, y31 sc8 wa | Originally used by us to create a line that combines In(1)sc8 and the Rif11 mutation, renamed #94727 after an additional inversion was discovered | BDSC |
| #798 “backup copy” (BDSC) | In(1)sc8, y31 sc8 wa | Subline of #798, retaining the original genotype | BDSC | |
| #798 2020 | Sub-lines created in 2020 based on the #798 “main copy” flies obtained from BDSC | |||
| #94727 (BDSC) | Renamed sub-line #798 main copy after detection of a new spontaneous inversion In(1)19EHet | In(1)sc8, In(1)19EHet, y31 sc8 wa | BDSC | |
| #798 (BDSC) | The BDSC stock with In(1)sc8, y31 sc8 wa genotype. After the discovery of In(1)19EHet in some flies, the line was cleared of carriers of additional inversion | In(1)sc8, y31 sc8 wa | BDSC | |
| In(1)sc8 + 19EHet; Rif11l | A line obtained by introducing a second chromosome from line Rif11 into line #798 “main copy” | In(1)sc8, In(1)19EHet, y31 sc8 wa; Rif11l | A line created for the cytological analysis of the pericentromeric heterochromatin of the X chromosome. A new inversion In(1)19EHet was discovered in it for the first time | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnikova, T.D.; Klenov, M.S.; Nokhova, A.R.; Lavrov, S.A.; Pokholkova, G.V.; Schubert, V.; Maltseva, S.V.; Cook, K.R.; Dixon, M.J.; Zhimulev, I.F. A Spontaneous Inversion of the X Chromosome Heterochromatin Provides a Tool for Studying the Structure and Activity of the Nucleolus in Drosophila melanogaster. Cells 2022, 11, 3872. https://doi.org/10.3390/cells11233872
Kolesnikova TD, Klenov MS, Nokhova AR, Lavrov SA, Pokholkova GV, Schubert V, Maltseva SV, Cook KR, Dixon MJ, Zhimulev IF. A Spontaneous Inversion of the X Chromosome Heterochromatin Provides a Tool for Studying the Structure and Activity of the Nucleolus in Drosophila melanogaster. Cells. 2022; 11(23):3872. https://doi.org/10.3390/cells11233872
Chicago/Turabian StyleKolesnikova, Tatyana D., Mikhail S. Klenov, Alina R. Nokhova, Sergey A. Lavrov, Galina V. Pokholkova, Veit Schubert, Svetlana V. Maltseva, Kevin R. Cook, Michael J. Dixon, and Igor F. Zhimulev. 2022. "A Spontaneous Inversion of the X Chromosome Heterochromatin Provides a Tool for Studying the Structure and Activity of the Nucleolus in Drosophila melanogaster" Cells 11, no. 23: 3872. https://doi.org/10.3390/cells11233872