A Segmental Approach from Molecular Profiling to Medical Imaging to Study Bicuspid Aortic Valve Aortopathy
Abstract
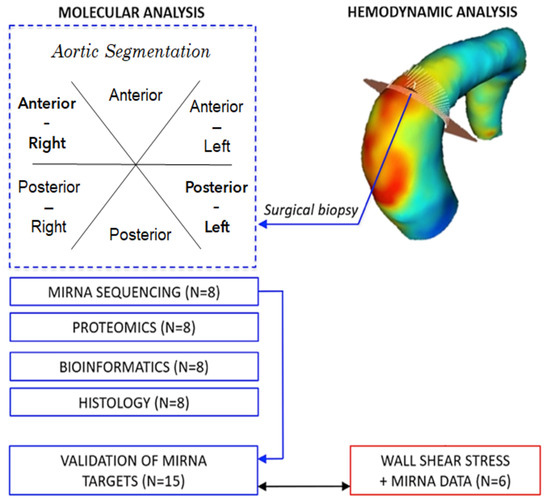
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Collection
2.3. Molecular Analyses
2.3.1. Next-Generation RNA Sequencing and Automated miRCURY LNA miRNA PCR Assay
2.3.2. Mass-Spectrometry Analysis
2.4. Bioinformatic Analysis
2.5. Histomorphometric Analysis
2.6. Haemodynamic Analysis
2.7. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Molecular Analysis
3.3. Bioinformatic Analysis
3.4. Histomorphometric Analysis
3.5. Haemodynamic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Patient Population
Data Collection
MiRNA Data Analysis
- (1)
- ΔCq = Cq(averaged normalisers)—Cq(miRNA of interest)
- (2)
- ΔΔCq = ΔCq(dilated segment)—ΔCq(non-dilated segment)
- (3)
- If ΔΔCq is positive, then FC = 2ΔΔCq
- (4)
- If ΔΔCq is negative, then FC = −[2(−ΔΔCq)]
Whole List of miRNAs from NGS Analysis
Whole List of Proteins from Tandem Mass Spectrometer Analysis

References
- Verma, S.; Siu, S.C. Aortic Dilatation in Patients with Bicuspid Aortic Valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, N.; Smelt, J.; Jahangiri, M. Bicuspid aortic valve aortopathy: Genetics, pathophysiology and medical therapy. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Peng, J.; Ren, Z.; He, N.Y.; Li, Q.; Zhao, X.S.; Wang, M.M.; Wen, H.Y.; Tang, Z.H.; Jiang, Z.S.; et al. Functional regulatory roles of microRNAs in atherosclerosis. Clin. Chim. Acta 2016, 460, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Alajbegovic, A.; Holmberg, J.; Albinsson, S. Molecular Regulation of Arterial Aneurysms: Role of Actin Dynamics and microRNAs in Vascular Smooth Muscle. Front. Physiol. 2017, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, J.S.; Hajira, A.; Lopez, M.A.; Boriek, A.M. Genome-wide Mechanosensitive MicroRNA (MechanomiR) Screen Uncovers Dysregulation of Their Regulatory Networks in the mdm Mouse Model of Muscular Dystrophy. J. Biol. Chem. 2015, 290, 24986–25011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzardi, D.G.; Barker, A.J.; van Ooij, P.; Malaisrie, S.C.; Puthumana, J.J.; Belke, D.D.; Mewhort, H.E.; Svystonyuk, D.A.; Kang, S.; Verma, S.; et al. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights from Wall Shear Stress Mapping. J. Am. Coll. Cardiol. 2015, 66, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Kiema, M.; Sarin, J.K.; Kauhanen, S.P.; Torniainen, J.; Matikka, H.; Luoto, E.S.; Jaakkola, P.; Saari, P.; Liimatainen, T.; Vanninen, R.; et al. Wall Shear Stress Predicts Media Degeneration and Biomechanical Changes in Thoracic Aorta. Front. Physiol. 2022, 13, 934941. [Google Scholar] [CrossRef]
- Albinsson, S.; Della Corte, A.; Alajbegovic, A.; Krawczyk, K.K.; Bancone, C.; Galderisi, U.; Cipollaro, M.; De Feo, M.; Forte, A. Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microrna signatures in mildly dilated ascending aorta. Heart Vessel. 2017, 32, 750–767. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, P.; Hartmann, N.; Baeriswy, L.; Andreasen, D.; Bernard, N.; Chen, C.; Cheo, D.; D’Andrade, P.; DeMayo, M.; Dennis, L.; et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nat. Methods 2014, 11, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.M. miR-128-3p Is a Novel Regulator of Vascular Smooth Muscle Cell Phenotypic Switch and Vascular Diseases (vol 126, e120, 2020). Circ. Res. 2020, 127, e120–e135. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Herrera-Morales, K.Y.; Perez-Torres, I. Participation of Arachidonic Acid Metabolism in the Aortic Aneurysm Formation in Patients with Marfan Syndrome. Front. Physiol. 2018, 9, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef] [Green Version]
- Araujo, N.N.F.; Lin-Wang, H.T.; Germano, J.F.; Farsky, P.S.; Feldman, A.; Rossi, F.H.; Izukawa, N.M.; Higuchi, M.L.; Savioli Neto, F.; Hirata, M.H.; et al. Dysregulation of microRNAs and target genes networks in human abdominal aortic aneurysm tissues. PLoS ONE 2019, 14, e0222782. [Google Scholar] [CrossRef]
- Zalewski, D.P.; Ruszel, K.P.; Stepniewski, A.; Galkowski, D.; Bogucki, J.; Komsta, L.; Kolodziej, P.; Chmiel, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulation of microRNA Modulatory Network in Abdominal Aortic Aneurysm. J. Clin. Med. 2020, 9, 1974. [Google Scholar] [CrossRef]
- Ikonomidis, J.S.; Ivey, C.R.; Wheeler, J.B.; Akerman, A.W.; Rice, A.; Patel, R.K.; Stroud, R.E.; Shah, A.A.; Hughes, C.G.; Ferrari, G.; et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J. Thorac. Cardiovasc. Surg. 2013, 145, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Girdauskas, E.; Neumann, N.; Petersen, J.; Sequeira-Gross, T.; Naito, S.; von Stumm, M.; von Kodolitsch, Y.; Reichenspurner, H.; Zeller, T. Expression Patterns of Circulating MicroRNAs in the Risk Stratification of Bicuspid Aortopathy. J. Clin. Med. 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.A. MicroRNA detection in the pathogenesis of BAV-associated aortopathy-mediated vascular remodelling through EndMT/EMT. J. Intern. Med. 2019, 285, 115–117. [Google Scholar] [CrossRef]
- Li, Y.; Maegdefessel, L. Non-coding RNA Contribution to Thoracic and Abdominal Aortic Aneurysm Disease Development and Progression. Front. Physiol. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, F.; Doldo, E.; Pisano, C.; Scioli, M.G.; Centofanti, F.; Proietti, G.; Falconi, M.; Sangiuolo, F.; Ferlosio, A.; Ruvolo, G.; et al. Specific miRNA and Gene Deregulation Characterize the Increased Angiogenic Remodeling of Thoracic Aneurysmatic Aortopathy in Marfan Syndrome. Int. J. Mol. Sci. 2020, 21, 6886. [Google Scholar] [CrossRef] [PubMed]
- Oceandy, D.; Amanda, B.; Ashari, F.Y.; Faizah, Z.; Aziz, M.A.; Stafford, N. The Cross-Talk Between the TNF- and RASSF-Hippo Signalling Pathways. Int. J. Mol. Sci. 2019, 20, 2346. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Bao, Q.; Yan, M.; Liang, J.; Zhu, Y.; Wang, C.; Ai, D. The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br. J. Pharmacol. 2018, 175, 1354–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korf-Klingebiel, M.; Reboll, M.R.; Grote, K.; Schleiner, H.; Wang, Y.; Wu, X.K.; Klede, S.; Mikhed, Y.; Bauersachs, J.; Klintschar, M.; et al. Heparan Sulfate-Editing Extracellular Sulfatases Enhance VEGF Bioavailability for Ischemic Heart Repair. Circ. Res. 2019, 125, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Du, P.Z.; Dai, F.J.; Chang, Y.W.; Wei, C.Y.; Yan, J.; Li, J.M.; Liu, X.B. Role of miR-199b-5p in regulating angiogenesis in mouse myocardial microvascular endothelial cells through HSF1/VEGF pathway. Environ. Toxicol. Pharmacol. 2016, 47, 142–148. [Google Scholar] [CrossRef]
- Xu, B.; Iida, Y.; Glover, K.J.; Ge, Y.; Wang, Y.; Xuan, H.; Hu, X.; Tanaka, H.; Wang, W.; Fujimura, N.; et al. Inhibition of VEGF (Vascular Endothelial Growth Factor)-A or its Receptor Activity Suppresses Experimental Aneurysm Progression in the Aortic Elastase Infusion Model. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1652–1666. [Google Scholar] [CrossRef]
- Holderfield, M.T.; Hughes, C.C.W. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ. Res. 2008, 102, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of Flow-Sensitive microRNAs in Endothelial Dysfunction and Atherosclerosis Mechanosensitive Athero-miRs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef] [Green Version]
- Sophocleous, F.; Milano, E.G.; Pontecorboli, G.; Chivasso, P.; Caputo, M.; Rajakaruna, C.; Bucciarelli-Ducci, C.; Emanueli, C.; Biglino, G. Enlightening the Association between Bicuspid Aortic Valve and Aortopathy. J. Cardiovasc. Dev. Dis. 2018, 5, 21. [Google Scholar] [CrossRef]
- Girdauskas, E.; Borger, M.A.; Secknus, M.A.; Girdauskas, G.; Kuntze, T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur. J. Cardio-Thorac. Surg. 2011, 39, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Padang, R.; Bannon, P.G.; Jeremy, R.; Richmond, D.R.; Semsarian, C.; Vallely, M.; Wilson, M.; Yan, T.D. The genetic and molecular basis of bicuspid aortic valve associated thoracic aortopathy: A link to phenotype heterogeneity. Ann. Cardiothorac. Surg. 2013, 2, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Atkins, S.K.; Sucosky, P. Etiology of bicuspid aortic valve disease: Focus on hemodynamics. World J. Cardiol. 2014, 6, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Saikrishnan, N.; Mirabella, L.; Yoganathan, A.P. Bicuspid aortic valves are associated with increased wall and turbulence shear stress levels compared to trileaflet aortic valves. Biomech. Model. Mechanobiol. 2015, 14, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.J.; Markl, M.; Burk, J.; Lorenz, R.; Bock, J.; Bauer, S.; Schulz-Menger, J.; von Knobelsdorff-Brenkenhoff, F. Bicuspid Aortic Valve Is Associated with Altered Wall Shear Stress in the Ascending Aorta. Circ.-Cardiovasc. Imaging 2012, 5, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Scardulla, F.; D’Acquisto, L.; Agnese, V.; Gentile, G.; Raffa, G.; Bellavia, D.; Pilato, M.; Pasta, S. Computational modeling of bicuspid aortopathy: Towards personalized risk strategies. J. Mol. Cell Cardiol. 2019, 131, 122–131. [Google Scholar] [CrossRef]
- Bissell, M.M.; Hess, A.T.; Biasiolli, L.; Glaze, S.J.; Loudon, M.; Pitcher, A.; Davis, A.; Prendergast, B.; Markl, M.; Barker, A.J.; et al. Aortic Dilation in Bicuspid Aortic Valve Disease Flow Pattern Is a Major Contributor and Differs with Valve Fusion Type. Circ.-Cardiovasc. Imaging 2013, 6, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Mahadevia, R.; Barker, A.J.; Schnell, S.; Entezari, P.; Kansal, P.; Fedak, P.W.; Malaisrie, S.C.; McCarthy, P.; Collins, J.; Carr, J.; et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014, 129, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.A.; Stroud, R.E.; O’Quinn, E.C.; Black, L.E.; Barth, J.L.; Elefteriades, J.A.; Bavaria, J.E.; Gorman, J.H.; Gorman, R.C.; Spinale, F.G.; et al. Selective MicroRNA Suppression in Human Thoracic Aneurysms Relationship of miR-29a to Aortic Size and Proteolytic Induction. Circ.-Cardiovasc. Genet. 2011, 4, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Borghini, A.; Foffa, I.; Pulignani, S.; Vecoli, C.; Ait-Ali, L.; Andreassi, M.G. miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing. Int. J. Mol. Sci. 2017, 18, 2498. [Google Scholar] [CrossRef]
- Yassine, N.M.; Shahram, J.T.; Body, S.C. Pathogenic Mechanisms of Bicuspid Aortic Valve Aortopathy. Front. Physiol. 2017, 8, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Huang, A.; Ferruzzi, J.; Mecham, R.P.; Starcher, B.C.; Tellides, G.; Humphrey, J.D.; Giordano, F.J.; Niklason, L.E.; Sessa, W.C. Inhibition of MicroRNA-29 Enhances Elastin Levels in Cells Haploinsufficient for Elastin and in Bioengineered Vessels-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 756–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef] [PubMed]






| Patient | Sex | Age at the Date of Study (yrs) | BSA (m2) | Type of Surgery | Type of Dilation | Degree of Dilation (echo mm) | Valve Phenotype | Aortic Regurgitation Severity | Aortic Stenosis Severity | Presence of Calcification | NGS, Proteomics, Histology | miRCURY LNA Assay | Haemodynamic Data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2 | F | 69 | 1.86 | AVR, AAo, AF | Anterior-Right | SoV:42 AAo:49 | RL | Moderate | None | None | ✓ | ✓ | ✓ |
| P3 | M | 68 | 1.98 | AVR, AAo | Anterior-Right | SoV:38 AAo:42 | RNC | None | Severe | Yes | ✓ | ✓ | ✓ |
| P6 | M | 58 | 2.18 | AVR, AAo | Anterior-Right | SoV:33 AAo:40 | RL | None | Moderate | Yes | ✓ | ✓ | |
| P7 | F | 66 | 1.84 | AVR, Arch | Anterior-Right | SoV:23 AAo:44 | RNC | Mild | Mild | None | ✓ | ✓ | |
| P8 | M | 59 | 1.88 | AVR, AAo/Root | Anterior-Right | SoV:44 AAo:41 | RL | None | Severe | Yes | ✓ | ✓ | |
| P9 | M | 65 | 2.35 | AVR, AAo, CAN | Anterior-Right | SoV:44 AAo:49 | RL | Moderate | None | None | ✓ | ✓ | |
| P14 | F | 57 | 1.55 | Ross | Anterior-Right | SoV:31 AAo:36 | RL | None | Severe | None | ✓ | ✓ | ✓ |
| P15 | F | 30 | 1.65 | Ozaki, AAo | Anterior-Right | SoV:37 AAo:37 | RL | Moderate | Mild | None | ✓ | ✓ | ✓ |
| P1 | M | 55 | 2.19 | AVR, AAo, AF, LAA | Overall | SoV:38 AAo:46 | RNC | Moderate | Moderate | None | ✓ | ✓ | |
| P4 | F | 32 | 1.9 | Ross | Anterior-Right | SoV:39 AAo:38 | RNC | Severe | Severe | Yes | ✓ | ||
| P5 | M | 34 | 1.92 | AVR, AAo | Anterior-Right | SoV:34 AAo:45 | RNC | Mild | Severe | Yes | ✓ | ||
| P10 | M | 70 | 1.78 | AVR, AAo | Overall | SoV:40 AAo:46 | RL | Mild | Severe | Yes | ✓ | ||
| P11 | M | 75 | 2.18 | Root | Anterior-Right | SoV:42 AAo:51 | RNC | Moderate | Moderate | Yes | ✓ | ||
| P12 | M | 56 | 1.99 | AVR, AAo | Anterior-Right | SoV:36 AAo:45 | N/A | Moderate | Severe | Yes | ✓ | ||
| P13 | M | 58 | 2.19 | Ozaki, AAo | Overall | SoV:46 AAo:53 | RNC | None | Severe | Yes | ✓ | ✓ |
| MiRNA Name | Dilated vs. Non-Dilated—FC | Dilated vs. Non-Dilated—Log2FC | Dilated vs. Non-Dilated—p-Value | Dilated vs. Non-Dilated—FDR p-Value |
|---|---|---|---|---|
| hsa-miR-1247-5p | 3.5892 | 1.8437 | <0.0001 | 0.0003 |
| hsa-miR-21-5p | 0.6667 | −0.5849 | <0.0001 | 0.0003 |
| hsa-miR-483-3p | 2.1387 | 1.0967 | <0.0001 | 0.0024 |
| hsa-miR-211-5p | 0.3308 | −1.5960 | <0.0001 | 0.0095 |
| hsa-miR-21-3p | 0.6068 | −0.7206 | <0.0001 | 0.0095 |
| hsa-miR-199b-5p | 2.1820 | 1.1256 | <0.0001 | 0.0095 |
| hsa-miR-128-3p | 0.6975 | −0.5197 | <0.0001 | 0.0095 |
| hsa-miR-150-5p | 1.8983 | 0.9247 | 0.0002 | 0.0431 |
| hsa-miR-488-3p | 2.7460 | 1.4573 | 0.0003 | 0.0615 |
| hsa-miR-375-3p | 2.5455 | 1.3480 | 0.0005 | 0.0872 |
| hsa-miR-210-3p | 0.6465 | −0.6294 | 0.0006 | 0.0996 |
| AR vs. PL | A + AR vs. P + PL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anterior-Right (N = 12) | Overall (N = 3) | Anterior-Right (N = 12) | Overall (N = 3) | |||||||
| MiRNA Targets NGS | p-Value | FC | Log2FC | FDR | p-Value | p-Value | FC | Log2FC | FDR | p-Value |
| miR-1247-5p | 0.110 | 1.647 | 0.720 | 0.154 | 0.750 | 0.470 | 1.291 | 0.368 | 0.470 | 0.470 |
| miR-21-5p | 0.339 | 0.919 | −0.121 | 0.396 | 0.250 | 0.034 | 0.835 | −0.261 | 0.060 | 0.250 |
| miR-21-3p | 0.791 | 1.030 | 0.043 | 0.791 | 0.500 | 0.380 | 0.869 | −0.202 | 0.444 | 0.500 |
| miR-199b-5p | 0.012 | 1.354 | 0.437 | 0.021 | 0.500 | 0.233 | 1.173 | 0.230 | 0.327 | 1.000 |
| miR-128-3p | 0.012 | 0.724 | −0.466 | 0.021 | 0.250 | <0.001 | 0.665 | −0.588 | 0.002 | 0.750 |
| miR-150-5p | 0.007 | 1.736 | 0.796 | 0.021 | 0.750 | 0.012 | 1.418 | 0.504 | 0.028 | 1.000 |
| miR-210-3p | 0.009 | 0.773 | −0.371 | 0.021 | 0.250 | <0.001 | 0.722 | −0.471 | 0.002 | 0.750 |
| Gene Target | Description | Functional Database | p-Value |
|---|---|---|---|
| MGAT1 | peptidyl-asparagine modification | Biological_Process | 0.0139 |
| PAPPA | response to gonadotropin | Biological_Process | 0.0144 |
| MGAT1; SULF1 | glycoprotein metabolic process | Biological_Process | 0.0150 |
| SULF1 | vascular-endothelial growth-factor production | Biological_Process | 0.0169 |
| PTGER3; SULF1 | muscle system process | Biological_Process | 0.0176 |
| MGAT1 | nucleotide sugar metabolic process | Biological_Process | 0.0184 |
| MGAT1 | amino sugar metabolic process | Biological_Process | 0.0188 |
| SULF1 | animal organ formation | Biological_Process | 0.0301 |
| SULF1 | nerve development | Biological_Process | 0.0379 |
| SULF1 | vascular-endothelial growth-factor receptor-signalling pathway | Biological_Process | 0.0427 |
| PTGER3 | phospholipase C-activating G-protein-coupled receptor-signalling pathway | Biological_Process | 0.0470 |
| STX5 | organelle disassembly | Biological_Process | 0.0470 |
| MGAT1 | catalytic activity, acting on a glycoprotein | Molecular_Function | 0.0114 |
| STX5 | SNAP receptor activity | Molecular_Function | 0.0149 |
| MGAT1 | manganese ion binding | Molecular_Function | 0.0301 |
| EIF5B | translation factor activity, RNA binding | Molecular_Function | 0.0427 |
| STX5 | SNARE binding | Molecular_Function | 0.0500 |
| STX5 | SNARE complex | Cellular_Component | 0.0233 |
| Gene Target | Description | p-Value |
|---|---|---|
| MGAT1 | N-glycan trimming and elongation in the cis-Golgi | 0.0027 |
| MGAT1; STX5 | transport to the Golgi and subsequent modification | 0.0039 |
| PTGER3 | prostanoid ligand receptors | 0.0048 |
| PTGER3 | eicosanoid ligand-binding receptors | 0.0080 |
| PTGER3 | small ligand GPCRs | 0.0102 |
| MGAT1; STX5 | asparagine N-linked glycosylation | 0.0104 |
| PTGER3 | relationship between inflammation, COX-2, and EGFR | 0.0133 |
| PAN3 | deadenylation of mRNA | 0.0133 |
| RASSF2 | Hippo signalling pathway | 0.0155 |
| STX5 | Cargo concentration in the ER | 0.0176 |
| STX5 | SNARE interactions in vesicular transport | 0.0181 |
| STX5 | intra-Golgi traffic | 0.0234 |
| PTGER3 | prostaglandin synthesis and regulation | 0.0239 |
| MGAT1 | N-glycan biosynthesis | 0.0260 |
| EIF5B | translation factors | 0.0286 |
| PTGER3 | regulation of lipolysis in adipocytes | 0.0286 |
| PAN3 | deadenylation-dependent mRNA decay | 0.0297 |
| STX5 | COPII-mediated vesicle transport | 0.0359 |
| PAN3 | RNA degradation | 0.0416 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sophocleous, F.; De Garate, E.; Bigotti, M.G.; Anwar, M.; Jover, E.; Chamorro-Jorganes, A.; Rajakaruna, C.; Mitrousi, K.; De Francesco, V.; Wilson, A.; et al. A Segmental Approach from Molecular Profiling to Medical Imaging to Study Bicuspid Aortic Valve Aortopathy. Cells 2022, 11, 3721. https://doi.org/10.3390/cells11233721
Sophocleous F, De Garate E, Bigotti MG, Anwar M, Jover E, Chamorro-Jorganes A, Rajakaruna C, Mitrousi K, De Francesco V, Wilson A, et al. A Segmental Approach from Molecular Profiling to Medical Imaging to Study Bicuspid Aortic Valve Aortopathy. Cells. 2022; 11(23):3721. https://doi.org/10.3390/cells11233721
Chicago/Turabian StyleSophocleous, Froso, Estefania De Garate, Maria Giulia Bigotti, Maryam Anwar, Eva Jover, Aranzazu Chamorro-Jorganes, Cha Rajakaruna, Konstantina Mitrousi, Viola De Francesco, Aileen Wilson, and et al. 2022. "A Segmental Approach from Molecular Profiling to Medical Imaging to Study Bicuspid Aortic Valve Aortopathy" Cells 11, no. 23: 3721. https://doi.org/10.3390/cells11233721