Inflammation: A Target for Treatment in Spinal Cord Injury
Abstract
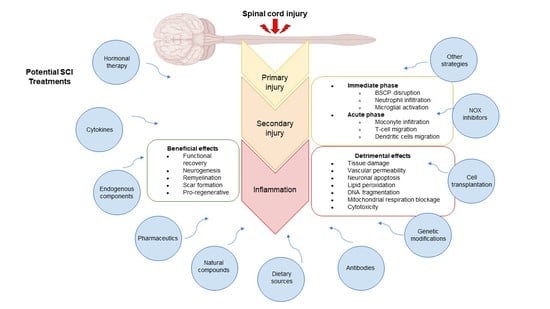
:1. Introduction
2. Main Pathological Events in SCI by Phase
2.1. Immediate Phase
2.2. Acute Phase
2.3. Intermediate and Chronic Phases
3. Main Inflammatory Events in SCI
3.1. Microglia Response
 ), mouse (
), mouse ( ), and human (
), and human ( ) Refs. [14,26,30,31,32,33].
) Refs. [14,26,30,31,32,33].
 ), mouse (
), mouse ( ), and human (
), and human ( ) Refs. [14,26,30,31,32,33].
) Refs. [14,26,30,31,32,33].
3.2. Neutrophils
3.3. Inflammasomes
3.4. Cytokines
3.5. Interferons
3.6. Macrophages
3.7. Dendritic Cells
3.8. Lymphocytes
4. Potential Therapies to Regulate the Inflammatory Response in SCI
4.1. Hormone Therapy
4.2. Cytokines and Interferons
4.3. Endogenous Components
4.4. Pharmaceuticals
4.5. Natural Compounds
4.6. Dietary Sources
4.7. Antibodies
4.8. Genetic Modifications
4.9. Cell Transplantation
- Mesenchymal stem cells (MSCs) reduce the inflammatory response in rodent models of SCI. MSCs transplanted directly into the SCI lesion site reduced the expression of multiple cytokines, including TNFα, IL-1β, IL-6, IL-2, IL-4, IL-12, IFN-α, and TGF-β1, among others. Transplantation of these cells also increased the expression of IL-4, IL-13, GM-CSF, and the ciliary neurotrophic factor. However, the reduced viability of the cells in the inflamed site of the SCI is the main drawback of direct MSCs transplantation [1].
- Bone marrow stromal cells (BMSCs) are considered an ideal cell source for treating SCI, as they can be donated for transplantation by the injured patient. Thus, immune repulsion can be prevented. BMSCs could be effectively guided to differentiate into neurons using the inverted colloidal crystal (ICC) scaffolds [138]. Yang et al. grafted two peptides to promote neurite outgrowth and cell attachment on the pore surface of ICC scaffolds and then evaluated the use of BMSBs on these peptide-modified ICC scaffolds as therapy in a rat model of contusion SCI. They observed a remarkable neuronal survival in animals treated with BMSCs in peptide-modified ICC scaffolds than in animals treated with directly administered BMSCs. In addition, they found decreased GFAP and TNF-α staining, meaning that this construct could inhibit or regulate glial scar formation and inflammation [138].
- Hematopoietic stem cells of umbilical cord blood have already proven helpful in treating various hematological and neurological disorders and types of cancer. Previously, Dasari et al. demonstrated that human umbilical cord blood stem cells (hUCBs) downregulate Fas and caspases, leading to functional recovery of rat hind limbs after SCI [139]. The authors further validated their data by analyzing the expression profiles of apoptotic genes in a model of moderate contusion SCI in rats transplanted with hUCBs. Their results showed efficient downregulation of these genes by hUCBs in the injured spinal cord. Treatment with hUCBs also downregulated the induced increase in TNF-α to basal levels, indicating their potential use as regulators of inflammation. Overall, this study showed that hUCBs could be an important therapeutic agent for the treatment of SCI [140].
- Neural stem cells (NSCs) can self-renew and generate neurons, oligodendrocytes, and astrocytes. NSCs immunoregulation and anti-inflammation effects have been demonstrated in vitro and in vivo. Transplantation of NSCs to the site of injury in the mouse contusion SCI model reduced neutrophils and regulated macrophage activation by inhibiting M1 macrophage activation. NSCs attenuated inflammatory cytokine mRNA levels, including TNF-α, IL-1β, IL-6 and IL-12. NSCs also inhibited the activation of bone marrow-derived macrophages, lessened the release cytokines such as TNF-α and IL-1β, and improved functional recovery after SCI [141].
- Dental stem cells (DSCs) are mesenchymal cells originating from the cranial neural crest. Yang et al. used a complete transection SCI model and injected DSCs into both sides of the injured spinal cord. Their results showed that some transplanted cells survived, differentiated into mature neurons and oligodendrocytes, and inhibited IL-1β expression to reduce inflammatory damage [142].
- The transplantation of stem cells from human exfoliated deciduous teeth (SHED) after SCI has also been explored. In a contusion SCI model, transplantation of SHEDs promoted functional recovery, decreased cystic cavity and glial scarring, and increased neurofilament density near the injury site. This study also demonstrated the ability of SHEDs to regulate inflammation in the context of SCI, as their transplantation reduced levels of the proinflammatory cytokine TNF-α [137].
- Olfactory ensheathing cells (OECs) are somatic cells whose transplantation into the injured spinal cord of rats reduced GFAP, IL-1β, and iNOS 14 days after the injury [104]. Schwann cells promote axonal regeneration in the peripheral nervous system and represent another somatic cell type whose use could repair the CNS after SCI [143]. After SCI, the co-transplantation of OECs and Schwann cells increased IL-4 and decreased IFN-γ levels, simultaneously reducing the cystic cavity area and improving motor functions [88].
4.10. NOX2 Inhibitors
4.11. Other Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, H.; Chen, X.; Tian, M.; Zhou, J.; Ouyang, H.; Zhang, Z. Regulation of inflammatory cytokines for spinal cord injury repair through local delivery of therapeutic agent. Adv. Sci. 2018, 5, 1800529. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, P.E.; Patil, A.A.; Chamczuk, A.J.; Agrawal, D.K. Hormonal therapy in traumatic spinal cord injury. Am. J. Transl. Res. 2017, 9, 3881–3895. [Google Scholar] [PubMed]
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.S.; Claus, C.F.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2020, 43, 425–441. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Kong, X.; Gao, J. Macrophage polarisation: A key event in the secondary phase of acute spinal cord injury. Cell. Mol. Med. 2016, 21, 941–954. [Google Scholar] [CrossRef]
- Riegger, T.; Conrad, S.; Schluesener, H.J.; Kaps, H.P.; Badke, A.; Baron, C.; Gerstein, J.; Dietz, K.; Abdizahdeh, M.; Schwab, J.M. Immune depression syndrome following human spinal cord injury (SCI): A pilot study. Neuroscience 2009, 158, 1194–1199. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef]
- Ding, L.; Fu, W.J.; Di, H.Y.; Zhang, X.M.; Lei, Y.T.; Chen, K.Z.; Wang, T.; Wu, H.F. Expression of long non-coding RNAs in complete transection spinal cord injury: A transcriptomic analysis. Neural Regen. Res. 2020, 15, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Miller, O.; Yovel, G.; Rosenzweig, N.; London, A.; Ruckh, J.; Kim, K.W.; Klein, E.; Kalchenko, V.; Bendel, P.; et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013, 38, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Lukacova, N.; Kisucka, A.; Kiss Bimbova, K.; Bacova, M.; Ileninova, M.; Kuruc, T.; Galik, J. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int. J. Mol. Sci. 2021, 22, 13577. [Google Scholar] [CrossRef]
- Trivedi, A.; Olivas, A.D.; Noble-Haeusslein, L.J. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin. Neurosci. Res. 2006, 6, 283–292. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale, M.S.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129 Pt 12, 3249–3269. [Google Scholar] [CrossRef]
- Popovich, P.G.; Wei, P.; Stokes, B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997, 377, 443–464. [Google Scholar] [CrossRef]
- Lin, Z.H.; Wang, S.Y.; Chen, L.L.; Zhuang, J.Y.; Ke, Q.F.; Xiao, D.R.; Lin, W.P. Methylene blue mitigates acute neuroinflammation after spinal cord injury through inhibiting NLRP3 inflammasome activation in microglia. Front. Cell. Neurosci. 2017, 11, 391. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, W.K.; Lu, C.Y.; Tsai, C.C.; Lai, H.L.; Lin, H.Y.; Guo, S.E.; Wu, L.M.; Chen, C.I. Mediating effects of social support and self-concept on depressive symptoms in adults with spinal cord injury. Spinal Cord 2015, 53, 413–416. [Google Scholar] [CrossRef]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Little, J.W.; Tessler, A.; Burns, A.S. Spinal shock revisited: A four-phase model. Spinal Cord 2004, 42, 383–395. [Google Scholar] [CrossRef]
- Xun, C.; Mamat, M.; Guo, H.; Mamati, P.; Sheng, J.; Zhang, J.; Xu, T.; Liang, W.; Cao, R.; Sheng, W. Tocotrienol alleviates inflammation and oxidative stress in a rat model of spinal cord injury via suppression of transforming growth factor-β. Exp. Ther. Med. 2017, 14, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Jing, N.; Fang, B.; Wang, Z.L.; Ma, H. Remote ischemia preconditioning attenuates blood-spinal cord barrier breakdown in rats undergoing spinal cord ischemia-reperfusion injury: Associated with activation and upregulation of CB1 and CB2 receptors. Cell. Physiol. Biochem. 2017, 43, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Wu, J.; Stoica, B.A.; Loane, D.J. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. J. Pharmacol. 2016, 173, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Dubový, P. Cytokines and their implication in axon degeneration and regeneration following peripheral nerve injury. In Cytokine Effector Functions in Tissues, 1st ed.; Foti, M., Locati, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 9; pp. 139–148. [Google Scholar] [CrossRef]
- Bloom, O.; Herman, P.E.; Spungen, A.M. Systemic inflammation in traumatic spinal cord injury. Exp. Neurol. 2020, 325, 113143. [Google Scholar] [CrossRef] [PubMed]
- Buzoianu-Anguiano, V.; Torres-Llacsa, M.; Doncel-Pérez, E. Role of aldynoglia cells in neuroinflammatory and neuroimmune responses after spinal cord injury. Cells 2021, 10, 2783. [Google Scholar] [CrossRef] [PubMed]
- Sroga, J.M.; Jones, T.B.; Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 2003, 462, 223–240. [Google Scholar] [CrossRef]
- Qu, W.S.; Tian, D.S.; Guo, Z.B.; Fang, J.; Zhang, Q.; Yu, Z.J.; Xie, M.J.; Zhang, H.Q.; Lü, J.G.; Wang, W. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J. Neuroinflamm. 2012, 9, 178. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Kim, C.C.; Nakamura, M.C.; Hsieh, C.L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflamm. 2016, 13, 117. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133 Pt 2, 433–447. [Google Scholar] [CrossRef]
- Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006, 494, 578–594. [Google Scholar] [CrossRef]
- Zrzavy, T.; Schwaiger, C.; Wimmer, I.; Berger, T.; Bauer, J.; Butovsky, O.; Schwab, J.M.; Lassmann, H.; Höftberger, R. Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain 2021, 144, 144–161. [Google Scholar] [CrossRef]
- Chio, J.; Xu, K.J.; Popovich, P.; David, S.; Fehlings, M.G. Neuroimmunological therapies for treating spinal cord injury: Evidence and future perspectives. Exp. Neurol. 2021, 341, 113704. [Google Scholar] [CrossRef] [PubMed]
- Gilgun, Y.S.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood-brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef]
- Kubick, N.; Henckell Flournoy, P.C.; Klimovich, P.; Manda, G.; Mickael, M.E. What has single-cell RNA sequencing revealed about microglial neuroimmunology? Immun. Inflamm. Dis. 2020, 8, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Milich, L.M.; Choi, J.S.; Ryan, C.; Cerqueira, S.R.; Benavides, S.; Yahn, S.L.; Tsoulfas, P.; Lee, J.K. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J. Exp. Med. 2021, 218, e20210040. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.; Uttam, S.; Ureña Guzmán, A.; Yaqubi, M.; Pacis, A.; Parisien, A.; Deamond, H.; Wong, C.; Rabau, O.; Brown, N.; et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 2022, 13, 843. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Zhou, L.; Shao, J.; Hu, X.; Xu, W.; Ren, Y.; Zhu, X.; Ge, W.; Zhang, K.; et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct. Target. Ther. 2022, 7, 65. [Google Scholar] [CrossRef]
- Nukolova, N.V.; Aleksashkin, A.D.; Abakumova, T.O.; Morozova, A.Y.; Gubskiy, I.L.; Kirzhanova, E.A.; Abakumov, M.A.; Chekhonin, V.P.; Klyachko, N.L.; Kabanov, A.V. Multilayer polyion complex nanoformulations of superoxide dismutase 1 for acute spinal cord injury. J. Control. Release 2018, 270, 226–236. [Google Scholar] [CrossRef]
- Louw, A.M.; Kolar, M.K.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Kjems, J.; Novikov, L.N. Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomedicine 2016, 12, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and clinical implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Tzekou, A.; Fehlings, M.G. Treatment of spinal cord injury with intravenous immunoglobulin G: Preliminary evidence and future perspectives. J. Clin. Immunol. 2014, 34 (Suppl. 1), S132–S138. [Google Scholar] [CrossRef]
- Stirling, D.P.; Liu, S.; Kubes, P.; Yong, V.W. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J. Neurosci. 2009, 29, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Taoka, Y.; Okajima, K.; Uchiba, M.; Murakami, K.; Kushimoto, S.; Johno, M.; Naruo, M.; Okabe, H.; Takatsuki, K. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997, 79, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Sawamura, S.; Takeda, K.; Orii, R.; Hanaoka, K. Anti-allodynic and anti-hyperalgesic effects of nociceptin receptor antagonist, JTC-801, in rats after spinal nerve injury and inflammation. Eur. J. Pharmacol. 2005, 510, 223–228. [Google Scholar] [CrossRef]
- Bao, F.; Dekaban, G.A.; Weaver, L.C. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J. Neurochem. 2005, 94, 1361–1373. [Google Scholar] [CrossRef]
- Urushitani, M.; Inoue, R.; Nakamizo, T.; Sawada, H.; Shibasaki, H.; Shimohama, S. Neuroprotective effect of cyclic GMP against radical-induced toxicity in cultured spinal motor neurons. J. Neurosci. Res. 2000, 61, 443–448. [Google Scholar] [CrossRef]
- Zgliczynski, J.M.; Stelmasynska, T.; Domanski, J. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim. Biophys. Acta 1971, 235, 419–424. [Google Scholar] [CrossRef]
- Khayrullina, G.; Bermudez, S.; Byrnes, K.R. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J. Neuroinflamm. 2015, 12, 172. [Google Scholar] [CrossRef]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yune, T.Y.; Lee, S.M.; Kim, S.J.; Park, H.K.; Oh, Y.J.; Kim, Y.C.; Markelonis, G.J.; Oh, T.H. Manganese superoxide dismutase induced by TNF-beta is regulated transcriptionally by NF-kappaB after spinal cord injury in rats. J. Neurotrauma 2004, 21, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.P.; Jiang, L.; Kang, K.; Fei, D.S.; Meng, X.L.; Nan, C.C.; Pan, S.H.; Zhao, M.R.; Zhao, M.Y. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int. Immunopharmacol. 2014, 20, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, M.; He, F.; Bian, Z.; He, Q.; Wang, X.; Yao, W.; Zhu, L. Neuroprotective effect of asiatic acid against spinal cord injury in rats. Life Sci. 2016, 157, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Johann, S.; Mehrabi, S.; Joghataei, M.T.; Hassanzadeh, G.; Kipp, M.; Beyer, C. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal cord injury model. Mol. Neurobiol. 2015, 53, 3063–3075. [Google Scholar] [CrossRef]
- Shang, A.J.; Yang, Y.; Wang, H.Y.; Tao, B.Z.; Wang, J.; Wang, Z.F.; Zhou, D.B. Spinal cord injury effectively ameliorated by neuroprotective effects of rosmarinic acid. Nutr. Neurosci. 2017, 20, 172–179. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, C.Y.; Bu, H.; Li, Z.; Li, B.; Sun, M.M.; Guo, Y.S.; Zhang, L.; Ren, W.B.; Fan, Z.L.; et al. The neuroprotective potential of phase II enzyme inducer on motor neuron survival in traumatic spinal cord injury in vitro. Cell. Mol. Neurobiol. 2008, 28, 769–779. [Google Scholar] [CrossRef]
- Wang, X.; de Rivero Vaccari, J.P.; Wang, H.; Diaz, P.; German, R.; Marcillo, A.E.; Keane, R.W. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J. Neurotrauma 2012, 29, 936–945. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.Y.; Choo, Y.Y.; Kim, O.; Tran, P.T.; Dao, C.T.; Min, B.S.; Lee, J.H. Sappanone A exhibits anti-inflammatory effects via modulation of Nrf2 and NF-κB. Int. Immunopharmacol. 2015, 28, 328–336. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef]
- Ning, N.; Dang, X.; Bai, C.; Zhang, C.; Wang, K. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J. Ethnopharmacol. 2012, 139, 504–512. [Google Scholar] [CrossRef]
- Burke, S.J.; Lu, D.; Sparer, T.E.; Karlstad, M.D.; Collier, J.J. Transcription of the gene encoding TNF-α is increased by IL-1β in rat and human islets and β-cell lines. Mol. Immunol. 2014, 62, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Sheikpranbabu, S.; Kalishwaralal, K.; Venkataraman, D.; Eom, S.H.; Park, J.; Gurunathan, S. Silver nanoparticles inhibit VEGF-and IL-1beta-induced vascular permeability via Src-dependent pathway in porcine retinal endothelial cells. J. Nanobiotechnol. 2009, 7, 8. [Google Scholar] [CrossRef]
- Wang, X.J.; Kong, K.M.; Qi, W.L.; Ye, W.L.; Song, P.S. Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol. Sin. 2005, 26, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Boato, F.; Rosenberger, K.; Nelissen, S.; Geboes, L.; Peters, E.M.; Nitsch, R.; Hendrix, S. Absence of IL-1β positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J. Neuroinflamm. 2013, 10, 792. [Google Scholar] [CrossRef]
- Schizas, N.; Andersson, B.; Hilborn, J.; Hailer, N.P. Interleukin-1 receptor antagonist promotes survival of ventral horn neurons and suppresses microglial activation in mouse spinal cord slice cultures. J. Neurosci. Res. 2014, 92, 1457–1465. [Google Scholar] [CrossRef]
- Akuzawa, S.; Kazui, T.; Shi, E.; Yamashita, K.; Bashar, A.H.; Terada, H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemic injury in rabbits. J. Vasc. Surg. 2008, 48, 694–700. [Google Scholar] [CrossRef]
- Zong, S.; Zeng, G.; Wei, B.; Xiong, C.; Zhao, Y. Beneficial effect of interleukin-1 receptor antagonist protein on spinal cord injury recovery in the rat. Inflammation 2012, 35, 520–526. [Google Scholar] [CrossRef]
- Chen, M.L.; Cao, H.; Chu, Y.X.; Cheng, L.Z.; Liang, L.L.; Zhang, Y.Q.; Zhao, Z.Q. Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: Multiple glial-neuronal dialogues in the rat spinal cord. J. Pain 2012, 13, 945–958. [Google Scholar] [CrossRef]
- Miyoshi, K.; Obata, K.; Kondo, T.; Okamura, H.; Noguchi, K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J. Neurosci. 2008, 28, 12775–12787. [Google Scholar] [CrossRef]
- Nakamura, M.; Okada, S.; Toyama, Y.; Okano, H. Role of IL-6 in spinal cord injury in a mouse model. Clin. Rev. Allergy Immunol. 2005, 28, 197–204. [Google Scholar] [CrossRef]
- Liu, S.G.; Ren, P.Y.; Wang, G.Y.; Yao, S.X.; He, X.J. Allicin protects spinal cord neurons from glutamate-induced oxidative stress through regulating the heat shock protein 70/inducible nitric oxide synthase pathway. Food Funct. 2015, 6, 321–330. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Adv. Sci. 2017, 4, 1700034. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Nobakht, M.; Bakhtiyari, M.; Beyer, C.; Kipp, M.; Baazm, M.; Joghataie, M.T. Stromal cell-derived factor-1 alpha (SDF-1α) improves neural recovery after spinal cord contusion in rats. Brain Res. 2012, 1473, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Shyu, W.C.; Lin, S.Z.; Yen, P.S.; Su, C.Y.; Chen, D.C.; Wang, H.J.; Li, H. Stromal cell-derived factor-1 alpha promotes neuroprotection, angiogenesis, and mobilisation/homing of bone marrow-derived cells in stroke rats. J. Pharmacol. Exp. Ther. 2008, 324, 834–849. [Google Scholar] [CrossRef]
- Gupta, R.C.; Seki, Y.; Yosida, J. Role of taurine in spinal cord injury. Curr. Neurovasc. Res. 2006, 3, 225–235. [Google Scholar] [CrossRef]
- Yune, T.Y.; Chang, M.J.; Kim, S.J.; Lee, Y.B.; Shin, S.W.; Rhim, H.; Kim, Y.C.; Shin, M.L.; Oh, Y.J.; Han, C.T.; et al. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J. Neurotrauma 2003, 20, 207–219. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Christensen, R.N.; Gensel, J.C.; Miller, B.A.; Sun, F.; Beattie, E.C.; Bresnahan, J.C.; Beattie, M.S. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 2008, 28, 11391–11400. [Google Scholar] [CrossRef]
- Tolosa, L.; Caraballo-Miralles, V.; Olmos, G.; Lladó, J. TNF-α potentiates glutamate-induced spinal cord motoneuron death via NF-κB. Mol. Cell. Neurosci. 2011, 46, 176–186. [Google Scholar] [CrossRef]
- Sharma, H.S.; Winkler, T.; Stålberg, E.; Gordh, T.; Alm, P.; Westman, J. Topical application of TNF-alpha antiserum attenuates spinal cord trauma-induced edema formation, microvascular permeability disturbances and cell injury in the rat. Acta Neurochir. Suppl. 2003, 86, 407–413. [Google Scholar] [CrossRef]
- Bao, G.; Li, C.; Qi, L.; Wang, N.; He, B. Tetrandrine protects against oxygen-glucose-serum deprivation/reoxygenation-induced injury via PI3K/AKT/NF-κB signaling pathway in rat spinal cord astrocytes. Biomed. Pharmacother. 2016, 84, 925–930. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, L.; Hou, Y.; Si, M.; Zhao, Y.P.; Nie, L. Plumbagin protects against spinal cord injury-induced oxidative stress and inflammation in Wistar rats through Nrf-2 upregulation. Drug Res. 2015, 65, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 2004, 19, 3375–3381. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, F.; Chen, G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Duan, Z.; Chen, K.; Liu, Z.; Zhang, L.; Yao, D.; Li, B. The effects of co-transplantation of olfactory ensheathing cells and Schwann cells on local inflammation environment in the contused spinal cord of rats. Mol. Neurobiol. 2017, 54, 943–953. [Google Scholar] [CrossRef]
- Yaguchi, M.; Ohta, S.; Toyama, Y.; Kawakami, Y.; Toda, M. Functional recovery after spinal cord injury in mice through activation of microglia and dendritic cells after IL-12 administration. J. Neurosci. Res. 2008, 86, 1972–1980. [Google Scholar] [CrossRef]
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, B.T.; Brennan, F.H.; Popovich, P.G. The spleen as a neuroimmune interface after spinal cord injury. J. Neuroimmunol. 2018, 321, 1–11. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.S.; Marbourg, J.M.; Brennan, F.H.; Mifflin, K.A.; Hall, J.C.E.; Jiang, R.R.; Mo, X.M.; Karunasiri, M.; Burke, M.H.; Dorrance, A.M.; et al. Spinal cord injury causes chronic bone marrow failure. Nat. Commun. 2020, 11, 3702. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Basso, D.M.; Sodhi, A.; Pan, J.P.; Hart, R.P.; MacCallum, R.C.; Lee, S.; Whitacre, C.C.; Popovich, P.G. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: Implications for autoimmune vaccine therapy. J. Neurosci. 2002, 22, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef]
- Jones, T.B.; Ankeny, D.P.; Guan, Z.; McGaughy, V.; Fisher, L.C.; Basso, D.M.; Popovich, P.G. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J. Neurosci. 2004, 24, 3752–3761. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Popovich, P.G. Central nervous system and non-central nervous system antigen vaccines exacerbate neuropathology caused by nerve injury. Eur. J. Neurosci. 2007, 25, 2053–2064. [Google Scholar] [CrossRef]
- Schwartz, M.; Kipnis, J. Protective autoimmunity: Regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol. Med. 2001, 7, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, J.; Mizrahi, T.; Hauben, E.; Shaked, I.; Shevach, E.; Schwartz, M. Neuroprotective autoimmunity: Naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc. Natl. Acad. Sci. USA 2002, 99, 15620–15625. [Google Scholar] [CrossRef]
- Pishva, A.; Akbari, M.; Farahabadi, A.; Arabkheradmand, A.; Beyer, C.; Dashti, N.; Moradi, F.; Hassanzadeh, G. Effect of estrogen therapy on TNF-α and iNOS gene expression in spinal cord injury model. Acta Med. Iran. 2016, 54, 296–301. [Google Scholar]
- Farahabadi, A.; Akbari, M.; Amini Pishva, A.; Zendedel, A.; Arabkheradmand, A.; Beyer, C.; Dashti, N.; Hassanzadeh, G. Effect of progesterone therapy on TNF-α and iNOS gene expression in spinal cord injury model. Acta Med. Iran. 2016, 54, 345–351. [Google Scholar]
- Yang, Z.; Xie, W.; Ju, F.; Khan, A.; Zhang, S. In vivo two-photon imaging reveals a role of progesterone in reducing axonal dieback after spinal cord injury in mice. Neuropharmacology 2017, 116, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yune, T.Y. Ghrelin inhibits oligodendrocyte cell death by attenuating microglial activation. Endocrinol. Metab. 2014, 29, 371–378. [Google Scholar] [CrossRef]
- López, R.V.; García, G.A.; Forés, J.; Vela, J.M.; Navarro, X.; Verdú, E. Transplanted olfactory ensheathing cells modulate the inflammatory response in the injured spinal cord. Neuron Glia Biol. 2004, 1, 201–209. [Google Scholar] [CrossRef]
- Theil, M.M.; Miyake, S.; Mizuno, M.; Tomi, C.; Croxford, J.L.; Hosoda, H.; Theil, J.; von Horsten, S.; Yokote, H.; Chiba, A.; et al. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J. Immunol. 2009, 183, 2859–2866. [Google Scholar] [CrossRef]
- Rey, M.; Coirini, H. Synthetic neurosteroids on brain protection. Neural Regen. Res. 2015, 10, 17–21. [Google Scholar] [CrossRef]
- Chuffa, L.G.; Lupi-Júnior, L.A.; Costa, A.B.; Amorim, J.P.; Seiva, F.R. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 2017, 118, 93–108. [Google Scholar] [CrossRef]
- Huie, J.R.; Baumbauer, K.M.; Lee, K.H.; Bresnahan, J.C.; Beattie, M.S.; Ferguson, A.R.; Grau, J.W. Glial tumor necrosis factor alpha (TNFα) generates metaplastic inhibition of spinal learning. PLoS ONE 2012, 7, e39751. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Smith, G.M.; Shine, H.D. Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury. Exp. Neurol. 2008, 209, 497–509. [Google Scholar] [CrossRef]
- Manickam, D.S.; Brynskikh, A.M.; Kopanic, L.J.; Sorgen, P.L.; Klyachko, N.L.; Batrakova, E.V.; Bronich, T.K.; Kabanov, A.V. Well-defined cross-linked antioxidant nanozymes for treatment of ischemic brain injury. J. Control. Release 2012, 162, 636–645. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, H.; Tao, Y.; Zhang, S.; Wang, J.; Feng, X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 2014, 266, 91–101. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, S.J.; Gorrie, C.A.; Velamoor, S.; Green, C.R.; Nicholson, L.F.B. Connexin43 mimetic peptide is neuroprotective and improves function following spinal cord injury. Neurosci. Res. 2013, 75, 256–267. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Stoica, B.; Riccio, A.; Ganji, A.P.; Loane, D.J.; Faden, A.I. Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann. Neurol. 2009, 66, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, G.; Bogel, R.; Schnitzler, C.; Utzmann, R. Meloxicam: Influence on arachidonic acid metabolism. Biochem. Pharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Hakan, T.; Toklu, H.Z.; Biber, N.; Celik, H.; Erzik, C.; Ogunc, A.V.; Cetinel, S.; Sener, G. Meloxicam exerts neuroprotection on spinal cord trauma in rats. Int. J. Neurosci. 2011, 121, 142–148. [Google Scholar] [CrossRef]
- Khalil, N.Y.; Aldosari, K.F. Meloxicam. Profiles Drug Subst. Excip. Relat. Methodol. 2020, 45, 159–197. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Guerra-Araiza, C.; Orozco-Suárez, S.; Salgado-Ceballos, H.; Feria-Romero, I.A.; Gallardo, J.M.; Orozco-Barrios, C.E. The importance of natural antioxidants in the treatment of spinal cord injury in animal models: An overview. Oxidative Med. Cell. Longev. 2019, 2019, 3642491. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Schültke, E.; Griebel, R.W.; Juurlink, B.H.J. Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord 2010, 48, 857–861. [Google Scholar] [CrossRef]
- Xiao, W.; Yu, A.; Liu, D.; Shen, J.; Xu, Z. Ligustilide treatment promotes functional recovery in a rat model of spinal cord injury via preventing ROS production. Int. J. Clin. Exp. Pathol. 2015, 8, 12005–12013. [Google Scholar]
- Hu, X.W.; Meng, D.; Fang, J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis 2008, 29, 2369–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2017, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Xie, Y.X.; Zhang, J.; Qiu, X.H.; Cheng, A.B.; Tian, L.; Ma, B.Y.; Hou, Y.B. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J. Recept. Signal Transduct. Res. 2015, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, H.L.; Hsieh, C.W.; Yang, Y.L.; Wung, B.S. Upregulation of NF-E2-related factor-2-dependent glutathione by carnosol provokes a cytoprotective response and enhances cell survival. Acta Pharmacol. Sin. 2011, 32, 62–69. [Google Scholar] [CrossRef]
- Wang, S.; Ren, D. Allicin protects traumatic spinal cord injury through regulating the HSP70/Akt/iNOS pathway in mice. Mol. Med. Rep. 2016, 14, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef]
- Nakajima, Y.; Osuka, K.; Seki, Y.; Gupta, R.C.; Hara, M.; Takayasu, M.; Wakabayashi, T. Taurine reduces inflammatory responses after spinal cord injury. J. Neurotrauma 2010, 27, 403–410. [Google Scholar] [CrossRef]
- Bao, F.; Chen, Y.; Dekaban, G.A.; Lynne, C.; Weaver, L.C. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J. Neurochem. 2004, 88, 1335–1344. [Google Scholar] [CrossRef]
- Guven, C.; Borcek, A.O.; Cemil, B.; Kurt, G.; Yildirim, Z.; Ucankus, N.L.; Kilic, N.; Ceviker, N. Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J. Clin. Neurosci. 2010, 17, 1563–1567. [Google Scholar] [CrossRef]
- Shaughnessy, A.F. Monoclonal antibodies: Magic bullets with a hefty price tag. BMJ 2012, 345, e8346. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, T.D.; Wu, H.; Langa, Y.; Li, D.Z.; Ni, S.F.; Lu, H.B.; Hu, J.Z. Synchrotron radiation micro-CT as a novel tool to evaluate the effect of agomir-210 in a rat spinal cord injury model. Brain Res. 2017, 1655, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, J. Targeted siRNA delivery reduces nitric oxide-mediated cell death after spinal cord injury. J. Nanobiotechnol. 2017, 15, 38. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010, 213 Pt 9, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V.; Mavilio, F. Interactions between retroviruses and the host cell genome. Mol. Ther. Methods Clin. Dev. 2017, 8, 31–41. [Google Scholar] [CrossRef]
- Nicola, F.C.; Rodrigues, L.P.; Crestani, T.; Quintiliano, K.; Sanches, E.F.; Willborn, S.; Aristimunha, D.; Boisserand, L.; Pranke, P.; Netto, C.A. Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz. J. Med. Biol. Res. 2016, 49, e5319. [Google Scholar] [CrossRef]
- Yang, J.T.; Kuo, Y.C.; Chiu, K.H. Peptide-modified inverted colloidal crystal scaffolds with bone marrow stromal cells in the treatment for spinal cord injury. Coll. Surf. B Biointerface 2011, 84, 198–205. [Google Scholar] [CrossRef]
- Dasari, V.R.; Spomar, D.G.; Li, L.; Gujrati, M.; Rao, J.S.; Dinh, D.H. Umbilical cord blood stem cell-mediated downregulation of Fas improves functional recovery of rats after spinal cord injury. Neurochem. Res. 2008, 33, 134–149. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Tsung, A.J.; Gondi, C.S.; Gujrati, M.; Dinh, D.H.; Rao, J.S. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J. Neurotrauma 2009, 26, 2057–2069. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, W.; Cao, K.; Wu, F.; Li, J.; Wang, G.; Li, H.; Lu, M.; Ren, Y.; He, X. Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int. J. Mol. Sci. 2016, 17, 1380. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Santamaria, A.J.; Benavides, F.D. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ Transpl. 2013, 18, 682–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauer, B.E.; Kornowski, R. Stem cell therapy in perspective. Circulation 2003, 107, 929–934. [Google Scholar] [CrossRef]
- Cooney, S.J.; Sabogal, B.S.L.; Byrnes, K.R. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflamm. 2013, 10, 155. [Google Scholar] [CrossRef]
- Cooney, S.J.; Zhao, Y.; Byrnes, K.R. Characterisation of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radic. Res. 2014, 48, 929–939. [Google Scholar] [CrossRef]
- Otto, A.; Fontaine, D.; Fontaine, J.; Berkenboom, G. Rosuvastatin treatment protects against nitrate-induced oxidative stress. J. Cardiovasc. Pharmacol. 2005, 148, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Mazzon, E.; Esposito, E.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. Effect of apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radic. Res. 2011, 45, 221–236. [Google Scholar] [CrossRef]
- Veronez, S.; Assis, L.; Campo, P.D.; Oliveira, F.D.; Castro, G.D.; Renno, A.C.M.; Medalha, C.C. Effects of different fluences of low-level laser therapy in an experimental model of spinal cord injury in rats. Lasers Med. Sci 2017, 32, 343–349. [Google Scholar] [CrossRef]
- Liu, F.T.; Xu, S.M.; Xiang, Z.H.; Li, X.N.; Li, J.; Yuan, H.B.; Sun, X.J. Molecular hydrogen suppresses reactive astrogliosis related to oxidative injury during spinal cord injury in rats. CNS Neurosci. Ther. 2014, 20, 778–786. [Google Scholar] [CrossRef]
- Choi, D.C.; Le, J.Y.; Moon, Y.J.; Kim, S.W.; Oh, T.H.; Yune, T.Y. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis. 2010, 39, 272–282. [Google Scholar] [CrossRef]
- Schmidt, E.; Raposo, P.; Vavrek, R.; Fouad, K. Inducing inflammation following subacute spinal cord injury in female rats: A double-edged sword to promote motor recovery. Brain. Behav. Immun. 2021, 93, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Torres-Espín, A.; Forero, J.; Fenrich, K.K.; Lucas-Osma, A.M.; Krajacic, A.; Schmidt, E.; Vavrek, R.; Raposo, P.; Bennett, D.J.; Popovich, P.G.; et al. Eliciting inflammation enables successful rehabilitative training in chronic spinal cord injury. Brain 2018, 141, 1946–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkema, S.J.; Hillyer, J.; Schmidt-Read, M.; Ardolino, E.; Sisto, S.A.; Behrman, A.L. Locomotor training as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef] [Green Version]


| Time after SCI | |||||
|---|---|---|---|---|---|
| Immediate | ≤2 h | ≤48 h | 2–13 Days | 2–6 Weeks | ≥6 Months |
| Primary Injury | Secondary Injury | ||||
| Immediate Phase | Acute Phase | Subacute Phase | Intermediate Phase | Chronic Phase | |
|
|
|
|
|
|
| Therapy | Treatment | Model | Clinical Status | Pharmacological Activities | Regulation of Target Molecules | Functional and Neurological Recovery | Refs |
|---|---|---|---|---|---|---|---|
| Hormone therapy | ER agonists (estrogen, tamoxifen) | Traumatic SCI in rats | Pre-clinical | Anti-inflammatory | Reduction of TNF-α and iNOS expression | Improved BBB scores Improved MEP conductance Increase in quantity and diameter of axons | [3] |
| Progesterone | SCI in rats and mice | Pre-clinical | Promyelinating, anti-inflammatory, and neuroprotective effects | Reduction of TNF-α and iNOS expression Downregulation of MCP-1, IL-1β, activated caspase-3, and GFAP | Improved motor function and histological outcomes Reduced axonal dieback and neuronal death | [101,102] | |
| Ghrelin | Autoimmune encephalomyelitis | Pre-clinical | Anti-inflammatory | Reduction of TNF-α, IL-1β, and IL-6 levels | Inhibited oligodendrocyte cell death Attenuated microglial activation | [103,105] | |
| Cytokines | TNF-α | Traumatic injury in rats | Pre-clinical | Antioxidant | Increased MnSOD activity Activation and nuclear translocation of NF-κB | — | [53] |
| IL-12 | SCI in female mice | Pre-clinical | Functional recovery | Increased BDNF expression | Improved BBB scores Increased remyelination Induced neurogenesis | [89] | |
| SDF1α | SCI in male rats | Pre-clinical | Anti-inflammatory Anti-apoptosis Neuroprotection | Reduction of NLRP3, ASC, TNF-α, IL-1β, IL-18, and caspase-1 levels | Improved functional long-term recovery Attenuation of the inflammasome complex | [56] | |
| Endogenous components | SOD1 | Moderate SCI in male rats | Pre-clinical | Anti-inflammatory Antioxidant | Decreased GFAP expression Reduction of ROS | Improved locomotor functions Decreased edema | [41] |
| Nec-1 | Contusion SCI in rats | Pre-clinical | Anti-inflammatory, antioxidant Kinase inhibitor Anti-necroptosis and anti-apoptosis | Reduction of TNF-α, IL-1β, and IL-6 levels and ROS | Reduced ischemia lesions Induces functional recovery and neuroprotection | [113] | |
| Peptide 5 | Moderate contusion SCI in rats | Pre-clinical | Anti-inflammatory | Reduction of TNF-α, IL-1β, and Cx43 levels Increased Cx43 phosphorylation | Improved functional recovery Increased motor neuron survival Reduced astrocytosis, activated microglia Prevented general secondary tissue damage | [114] | |
| EGFR inhibition by C225 and AG1478 | SCI in male rats | Pre-clinical | Inhibition of the EGFR/MAPK cascade Anti-inflammatory | Decreased IL-1β, TNF-α, CD11b, and GFAP | Reduced activation of microglia and astrocytes Attenuated tissue edema Improved morphological and functional recovery | [27] | |
| CHPG (mGluR5 agonist) | Moderate SCI in male rats | Pre-clinical | Attenuation of microglial-associated inflammation | Reduction of ED1, Iba-1, Galectin-3, NADPH oxidase components, TNF-α, iNOS | Improved functional motor recovery Reduction of lesion size | [115] | |
| Pharmaceuticals | Meloxicam | Contusion SCI in rats | Pre-clinical | Inhibition of COX-2 Antioxidant and anti-inflammatory | Reduction of MPO activity (neutrophil infiltration), lipid peroxidation, and DNA damage Inhibition of free radical generation | Ameliorated histological and neurological deterioration | [117] |
| Methylene blue | SCI in male rats | Pre-clinical | Anti-inflammatory, anti-apoptosis, and antioxidant Targeted mitochondria toxicity Inhibition of inflammasome formation | Decreased IL-1β and IL-18, ROS, and cleaved caspase-1 levels Reduction of NLRP3 and NLRC4 activation | Ameliorated hind limb locomotor function | [16] | |
| Natural compounds | Quercetin | Compression SCI in male rats | Pre-clinical | Antioxidant, anti-inflammatory, anti-carcinogenic Reduction of neutrophil recruitment | Reduction of MPO activity | Decreased white blood count in venous blood | [121] |
| Ligustilide | Transection SCI in rats | Pre-clinical | Neuroprotection Anti-cancer, anti-inflammatory, and analgesic properties | Prevention of ROS production Suppression of iNOS expression Reduction of IL-1β, TNF-α levels | Improved BBB scale Reduced recovery of coordination Expands blood vessels Inhibition of vascular smooth muscle cells proliferation | [122] | |
| Asiatic acid | SCI in female rats | Pre-clinical | Antioxidant and anti-inflammatory Inhibition of NLRP3 inflammasome activation | Suppression of MPO Reduction of IL-1β, IL-18, TNF-α, and IL-6 levels Upregulation of Nrf2/HO-1 levels | Increased BBB scores Reduced inclined plane scores | [55] | |
| Tetrandrine | OGSD/R-induced injury in rat spinal cord astrocytes | Pre-clinical | Anti-inflammatory, antioxidant, antitumor, anti-nociceptive, and antidepressant | Decreased TNF-α, IL-1β, and IL-6 accumulation | Attenuated oxidative stress in vitro | [82] | |
| PNS | Acute spinal cord IRI in rats | Pre-clinical | Anti-inflammation, anti-edema, and anti-apoptosis | Prevention of IL-1β, IL-10, and TNF-α increase | Increased BBB scores Retained neurons Restored neuronal morphology Reduced leukocytes activity | [62] | |
| Plumbagin | SCI in male rats | Pre-clinical | Anti-proliferative, chemo-preventive, anti-metastatic, anti-inflammatory, and analgesic | Downregulation of TNF-α, IL-1β Suppression of NF-kB expression Enhancement of Nrf-2 nuclear levels | Reduced ROS and lipid peroxidation Increased antioxidant pool | [83] | |
| Apigenin | Traumatic SCI in rats | Pre-clinical | Antioxidant, anti-inflammatory, and anti-apoptosis | Decreased IL-1β, TNF-α, and ICAM-1 levels | Increased BBB scores Reversed changes in MDA content, SOD, and GSH-Px activity | [86] | |
| Carnosol | SCI in rats | Pre-clinical | Antioxidant, anticancer, and anti-inflammatory properties Enhancement of Nrf2-related antioxidant defense | Downregulation of NF-κB and COX-2 levels Decreased TNF-α, IL-6, and IL-1β levels Upregulation of p-Akt and Nrf-2 levels | Enhancement in TAC Declined TOS Reduced histological damage | [125] | |
| Rosmarinic acid | SCI in male rats | Pre-clinical | Antioxidant and anti-inflammatory Anti-apoptosis | Downregulation of NF-κB, IL-6, IL-1β, TNF-α, and MCP-1 levels Upregulation of Nrf-2 expression | Improved motor function Decreased oxidative stress Enhanced antioxidant status Prevented neural apoptosis | [57] | |
| Tocotrienol | SCI in female rats | Pre-clinical | Anti-oxidative, anti-inflammatory, anti-apoptotic, and neuroprotection functions | Reduction of NF-κB p65 unit, TNF-α, IL-1β, and IL-6 levels Decreased iNOS expression and activity and NO production Inhibition of TGF-β, collagen type IV, and fibronectin expression | Improved BBB scores Reduced volume of grey matter contusions in injured spinal cords Prevented oxidative damage | [20] | |
| Dietary sources | Allicin | Traumatic SCI in mice | Pre-clinical | Anti-inflammatory, antibiotic, antioxidant, and antitumor | Reduction of NF-κB and TNF-α levels Inhibition of iNOS expression and ROS levels Increased CAT and SOD activity Increased HSP70 expression Elevated NADH levels | Increased BBB scores Reduced spinal cord water content | [127] |
| Taurine | SCI in mice | Pre-clinical | Anti-inflammatory | Decreased IL-6 and MPO levels and COX-2 expression | Reduced neutrophil accumulation Improved functional recovery in hind limbs | [129] | |
| Antibodies | mAb against P-selectin | Compression SCI in male rats | Pre-clinical | Anti-inflammatory | Inhibition of MPO activity P-selectin | Improved motor functions Attenuated intramedullary hemorrhages Decreased accumulation of neutrophils | [46] |
| mAb against the CD11d subunit of CD11d/CD18 | SCI in female rats | Pre-clinical | Reduction of neutrophil and macrophage infiltration Anti-apoptosis Anti-inflammatory and antioxidant | Decreased ED-1 and iNOS expression Reduction of MPO activity, protein nitrosylation, and lipid peroxidation Reduction of ROS, RNS, MDA, 4-HNE, and caspase-3 | Improved tissue preservation and neurological function Decreased intraspinal inflammation Reduced apoptosis and cell death | [48,130] | |
| Infliximab | IRI to the spinal cord in male rabbits | Pre-clinical | Reduces damage caused by ischemia-reperfusion injury improves biochemical and histological outcome | Decreased MDA, GSH, and AOPP levels Increased SOD activity | Improved Tarlov scores Reduced vascular proliferation, edema, and neuronal loss | [131] | |
| Genetic modifications | Agomir-210 | Contusion SCI in male rats | Pre-clinical | Anti-inflammatory Attenuation of apoptosis | Decreased Bax, TNFα, and IL-1β Upregulation of Bcl-2 and IL-10 | Promoted angiogenesis Attenuated the lesion size Improved functional recovery | [133] |
| siRNA-chitosan nanoparticles | Traumatic SCI in female mice Compression SCI in guinea pigs | Pre-clinical | Anti-apoptotic, antioxidant anti-inflammatory Limitation of lipid peroxidation | Reduction of iNOS and Bax expression Increased Bcl-2 expression and NO production | Restored nerve conduction | [134,135] | |
| miRNA-124-chitosan polyplex | Traumatic SCI in female rats | Pre-clinical | Anti-inflammatory Limitation of lipid peroxidation | Reduction of MHC-II, TNF-α, and ROS production | Modulated macrophage/microglia activation | [42,135] | |
| Lentivirus | SCI in rats | Pre-clinical | Anti-inflammatory Promotion of M2 polarisation Neurorepair | Decreased TNFα and IL-1 β expression Upregulation of IL-10 and IL-13 | Improved motor function | [1] | |
| Cell transplantation | MSCs | SCI in rodents | Pre-clinical | Anti-inflammatory | Reduction of TNF-α, IL-1β, IL-6, IL-2, IL-12, IFN-α, TGF-β1, MMP-9, CCL2, CCL5, and CCL10 expression Increased IL-4, IL-13, CCL5, GM-CSF, leptin, and ciliary neurotrophic factor levels | Promoted functional recovery Limited lesion volume Less scar tissue formation | [1] |
| BMSCs | SCI in male rats | Pre-clinical | Anti-inflammatory | Reduction of GFAP and TNF-α expression | Improves neuronal survival Reduced reactive gliosis | [138] | |
| hUCBs | SCI in male rats | Pre-clinical | Anti-apoptosis, anti-inflammatory | Downregulation of Fas expression Decreased caspases and TNF-α expression | Improved functional recovery of hind limbs Repaired spinal cord integrity | [139] | |
| NSCs | SCI in mice | Pre-clinical | Anti-inflammatory Immunoregulation | Attenuation of TNF-α, IL-1β, IL-6, and IL-12 mRNA levels Inhibition of iNOS expression | Improved BSA scores Reduced neutrophils Generation of neurons, oligodendrocytes, and astrocytes Inhibits the activation of M1 macrophages | [141] | |
| DSCs | SCI in female rats | Pre-clinical | Anti-inflammatory Neuroprotection and neuro-regeneration | Inhibition of IL-1β expression | Promoted functional recovery of hind limbs Ameliorated neural loss | [142] | |
| SHEDs | Contusion SCI in male rats | Pre-clinical | Anti-inflammatory | Reduction of TNF-α levels | Improved locomotor recovery Decreases cystic cavity area and glial scar formation | [137] | |
| OECs | Photochemical SCI in female rats | Pre-clinical | Anti-inflammatory, antioxidant | Reduction of GFAP activity, IL-1β, and iNOS levels | Reduced reactive gliosis Reduces cystic cavity area Improved neurological and electrophysiological recovery | [104] | |
| Co-transplantation of Schwann cells and OECs | SCI in female rats | Pre-clinical | Anti-inflammatory | Increased IL-4, IL-10, and IL-13 levels Decreased IFN-γ, IL-6 and TNF-α levels | Reduced cystic cavity area Improved motor functions | [88] | |
| NOX inhibitors | gp91ds-tat | SCI in male rats | Pre-clinical | Anti-inflammatory, antioxidant | Inhibition of NOX2 Suppression of IL-1β, IL-6, IL-12, and TNF-α, and other pro-inflammatory cytokines Reduction of ROS | Reduced neutrophil and macrophage/microglia invasion Reduced neuronal death | [146] |
| Apocynin | Compression SCI in male mice | Pre-clinical | Anti-inflammatory, antioxidant, anti-apoptotic | Blocking NADPH oxidase activation Attenuation of IL-1β, TNF-α, ICAM-1, P-selectin expression, and MPO activity Reduction of nitrotyrosine, poly-ADP-ribose, FAS ligand Prevention of Bax expression Reduction of NF-kB and P38MAPK levels Disinhibition of Bcl-2 expression Prevention of IκB-α degradation | Reduced adhesion molecule expression and neutrophil infiltration Decreased degree of tissue injury Ameliorated the loss of limb function | [148] | |
| Other strategies | PBM | SCI in female rats | Pre-clinical | Anti-inflammatory | Decreased CD68+ cells | Improved tactile sensitivity Promoted functional recovery Reduced lesion volume | [149] |
| CONPs | SCI in female rats | Pre-clinical | Anti-inflammatory, anti-apoptotic, anti-oxidative | Downregulation of iNOS, COX2, Nrf2, caspase 3, IL-1β, IL-6, and TNF-α levels Upregulation of IL-10 | Reduced cavity size Improved locomotor functions | [74] | |
| HrS | Contusion SCI in male rats | Pre-clinical | Anti-inflammatory | Attenuation of IL-1β, IL-6, and TNF-α release Decreased GFAP, m STAT3, and p-STAT3 expression Decreased ROS production | Suppressed reactive gliosis Alleviated the glial scar barrier Reduced axonal injury Improved locomotor function | [150] | |
| Acupuncture | Contusion SCI in male rats | Pre-clinical | Anti-inflammatory and anti-apoptotic | Attenuation of p38MAPK activation Inhibition of caspase-3 activation Reduction of TNF-α, IL-1β, IL-6, iNOS, COX-2, and MMP-9 expression | Improved functional recovery Reduced size of the lesion cavity Attenuates ischemia-induced cerebral infarction Enhances plasticity in CNS axons | [151] | |
| LPS + motor rehabilitation (high-intensity training) | Chronic SCI in female rats | Pre-clinical | Inflammatory Reopening of a plasticity period | — | Increases corticospinal axons sprouting into intermediate grey matter Enhanced motor recovery in forelimbs Regain of function | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freyermuth-Trujillo, X.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Orozco-Barrios, C.E.; Coyoy-Salgado, A. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells 2022, 11, 2692. https://doi.org/10.3390/cells11172692
Freyermuth-Trujillo X, Segura-Uribe JJ, Salgado-Ceballos H, Orozco-Barrios CE, Coyoy-Salgado A. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells. 2022; 11(17):2692. https://doi.org/10.3390/cells11172692
Chicago/Turabian StyleFreyermuth-Trujillo, Ximena, Julia J. Segura-Uribe, Hermelinda Salgado-Ceballos, Carlos E. Orozco-Barrios, and Angélica Coyoy-Salgado. 2022. "Inflammation: A Target for Treatment in Spinal Cord Injury" Cells 11, no. 17: 2692. https://doi.org/10.3390/cells11172692