Cell Biology of Spinal Cord Injury and Repair
Share This Topical Collection
Editors
 Prof. Dr. Shuxin Li
Prof. Dr. Shuxin Li
 Prof. Dr. Shuxin Li
Prof. Dr. Shuxin Li
E-Mail
Website
Collection Editor
Lewis Katz School of Medicine, Temple University, Philadelphia, PA 19122, USA
Interests: spinal cord injury; CNS injuries; cellular damage; neural repair; therapeutic strategies
 Prof. Dr. George Smith
Prof. Dr. George Smith
 Prof. Dr. George Smith
Prof. Dr. George Smith
E-Mail
Website
Collection Editor
Shriners Hospitals Pediatric Research Center (Center for Neural Repair and Rehabilitation), Department of Neural Sciences, Lewis Katz School of Medicine, Temple University, Philadelphia, PA 19122, USA
Interests: spinal cord injury
 Prof. Dr. Junfang Wu
Prof. Dr. Junfang Wu
 Prof. Dr. Junfang Wu
Prof. Dr. Junfang Wu
E-Mail
Website
Collection Editor
School of Medicine, University of Maryland, 655 W. Baltimore Street, Baltimore, MD 21201, USA
Interests: spinal cord injury; traumatic brain injury; aging; inflammation; neuroprotection; autophagy-lysosomal; neuropathic pain; Hv1/NOX2/ROS; TrkB.T1; microRNA; motor function; cognition; depression; neurons; astrocytes; microglia
Topical Collection Information
Dear Colleagues,
Spinal cord injury (SCI) usually causes complicated pathophysiology in the CNS and persistent functional deficits without a cure. Recently, researchers have made great progress in understanding secondary cellular injuries and in developing novel and effective neuroprotective and regenerative strategies for repairing the injured spinal cord. Scientists have also identified highly effective approaches for promoting cell survival, CNS regeneration, remyelination, neural circuit reconnections, and neuroplasticity after SCI and attempted to translate them into human use successfully. In this Topical Collection, we invite investigators to contribute original research or review articles that highlight recent important advances in the cell biology of SCI and neural repair, including novel mechanistic insights into neural damage and cell loss, regeneration failure and functional loss, and development of effective strategies to enhance CNS repair, cell survival, neuronal regeneration, remyelination, and functional recovery. Potential topics include but are not limited to the following:
- Cellular and molecular responses of neural cells to SCI and other CNS injuries, including neuroimmune responses, glial scar genesis, and glial–neuronal interactions;
- Novel mechanisms underlying cell damage/death, regeneration failure, and demyelination after SCI;
- Novel cellular targets and strategies for neuroprotection and for enhancing cell growth, neuronal regeneration, remyelination, and neuroplasticity after CNS injuries;
- Other promising therapeutic and translational strategies for repairing injured spinal cord or other CNS regions.
Prof. Dr. Shuxin Li
Prof. Dr. George Smith
Prof. Dr. Junfang Wu
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (23 papers)
Open AccessReview
Potential Roles of Specific Subclasses of Premotor Interneurons in Spinal Cord Function Recovery after Traumatic Spinal Cord Injury in Adults
by
Ana Dominguez-Bajo and Frédéric Clotman
Viewed by 344
Abstract
The differential expression of transcription factors during embryonic development has been selected as the main feature to define the specific subclasses of spinal interneurons. However, recent studies based on single-cell RNA sequencing and transcriptomic experiments suggest that this approach might not be appropriate
[...] Read more.
The differential expression of transcription factors during embryonic development has been selected as the main feature to define the specific subclasses of spinal interneurons. However, recent studies based on single-cell RNA sequencing and transcriptomic experiments suggest that this approach might not be appropriate in the adult spinal cord, where interneurons show overlapping expression profiles, especially in the ventral region. This constitutes a major challenge for the identification and direct targeting of specific populations that could be involved in locomotor recovery after a traumatic spinal cord injury in adults. Current experimental therapies, including electrical stimulation, training, pharmacological treatments, or cell implantation, that have resulted in improvements in locomotor behavior rely on the modulation of the activity and connectivity of interneurons located in the surroundings of the lesion core for the formation of detour circuits. However, very few publications clarify the specific identity of these cells. In this work, we review the studies where premotor interneurons were able to create new intraspinal circuits after different kinds of traumatic spinal cord injury, highlighting the difficulties encountered by researchers, to classify these populations.
Full article
►▼
Show Figures
Open AccessReview
Regenerative Potential of Injured Spinal Cord in the Light of Epigenetic Regulation and Modulation
by
Samudra Gupta, Suman Dutta and Subhra Prakash Hui
Cited by 4 | Viewed by 1830
Abstract
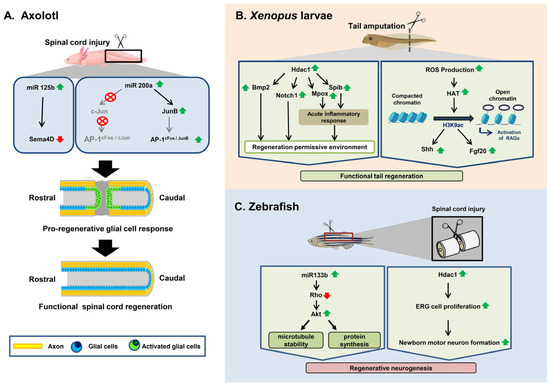
A spinal cord injury is a form of physical harm imposed on the spinal cord that causes disability and, in many cases, leads to permanent mammalian paralysis, which causes a disastrous global issue. Because of its non-regenerative aspect, restoring the spinal cord’s role
[...] Read more.
A spinal cord injury is a form of physical harm imposed on the spinal cord that causes disability and, in many cases, leads to permanent mammalian paralysis, which causes a disastrous global issue. Because of its non-regenerative aspect, restoring the spinal cord’s role remains one of the most daunting tasks. By comparison, the remarkable regenerative ability of some regeneration-competent species, such as some Urodeles (Axolotl),
Xenopus, and some teleost fishes, enables maximum functional recovery, even after complete spinal cord transection. During the last two decades of intensive research, significant progress has been made in understanding both regenerative cells’ origins and the molecular signaling mechanisms underlying the regeneration and reconstruction of damaged spinal cords in regenerating organisms and mammals, respectively. Epigenetic control has gradually moved into the center stage of this research field, which has been helped by comprehensive work demonstrating that DNA methylation, histone modifications, and microRNAs are important for the regeneration of the spinal cord. In this review, we concentrate primarily on providing a comparison of the epigenetic mechanisms in spinal cord injuries between non-regenerating and regenerating species. In addition, we further discuss the epigenetic mediators that underlie the development of a regeneration-permissive environment following injury in regeneration-competent animals and how such mediators may be implicated in optimizing treatment outcomes for spinal cord injurie in higher-order mammals. Finally, we briefly discuss the role of extracellular vesicles (EVs) in the context of spinal cord injury and their potential as targets for therapeutic intervention.
Full article
►▼
Show Figures
Open AccessReview
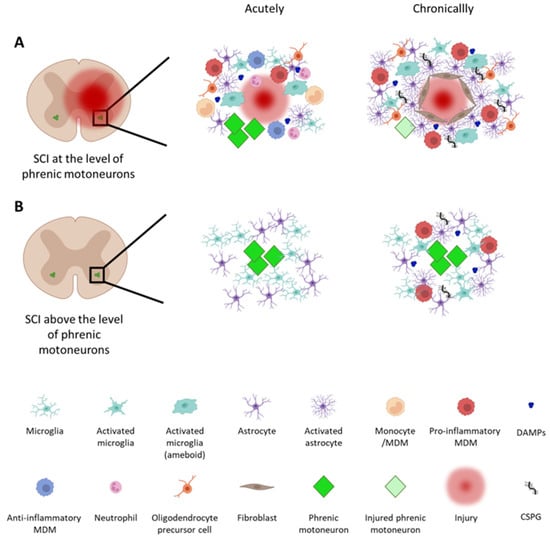
Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation
by
Pauline Michel-Flutot, Michael A. Lane, Angelo C. Lepore and Stéphane Vinit
Cited by 3 | Viewed by 1720
Abstract
High spinal cord injuries (SCIs) lead to permanent functional deficits, including respiratory dysfunction. Patients living with such conditions often rely on ventilatory assistance to survive, and even those that can be weaned continue to suffer life-threatening impairments. There is currently no treatment for
[...] Read more.
High spinal cord injuries (SCIs) lead to permanent functional deficits, including respiratory dysfunction. Patients living with such conditions often rely on ventilatory assistance to survive, and even those that can be weaned continue to suffer life-threatening impairments. There is currently no treatment for SCI that is capable of providing complete recovery of diaphragm activity and respiratory function. The diaphragm is the main inspiratory muscle, and its activity is controlled by phrenic motoneurons (phMNs) located in the cervical (C3–C5) spinal cord. Preserving and/or restoring phMN activity following a high SCI is essential for achieving voluntary control of breathing. In this review, we will highlight (1) the current knowledge of inflammatory and spontaneous pro-regenerative processes occurring after SCI, (2) key therapeutics developed to date, and (3) how these can be harnessed to drive respiratory recovery following SCIs. These therapeutic approaches are typically first developed and tested in relevant preclinical models, with some of them having been translated into clinical studies. A better understanding of inflammatory and pro-regenerative processes, as well as how they can be therapeutically manipulated, will be the key to achieving optimal functional recovery following SCIs.
Full article
►▼
Show Figures
Open AccessArticle
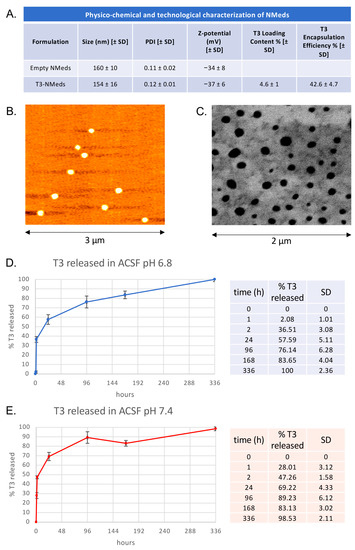
“Combo” Multi-Target Pharmacological Therapy and New Formulations to Reduce Inflammation and Improve Endogenous Remyelination in Traumatic Spinal Cord Injury
by
Marzia Moretti, Riccardo Caraffi, Luca Lorenzini, Ilaria Ottonelli, Michele Sannia, Giuseppe Alastra, Vito Antonio Baldassarro, Alessandro Giuliani, Jason Thomas Duskey, Maura Cescatti, Barbara Ruozi, Luigi Aloe, Maria Angela Vandelli, Luciana Giardino, Giovanni Tosi and Laura Calzà
Cited by 2 | Viewed by 2265
Abstract
Spinal cord injury (SCI) is characterized by a cascade of events that lead to sensory and motor disabilities. To date, this condition is irreversible, and no cure exists. To improve myelin repair and limit secondary degeneration, we developed a multitherapy based on nanomedicines
[...] Read more.
Spinal cord injury (SCI) is characterized by a cascade of events that lead to sensory and motor disabilities. To date, this condition is irreversible, and no cure exists. To improve myelin repair and limit secondary degeneration, we developed a multitherapy based on nanomedicines (NMeds) loaded with the promyelinating agent triiodothyronine (T3), used in combination with systemic ibuprofen and mouse nerve growth factor (mNGF). Poly-L-lactic-co-glycolic acid (PLGA) NMeds were optimized and loaded with T3 to promote sustained release. In vitro experiments confirmed the efficacy of T3-NMeds to differentiate oligodendrocyte precursor cells. In vivo rat experiments were performed in contusion SCI to explore the NMed biodistribution and efficacy of combo drugs at short- and long-term post-lesion. A strong anti-inflammatory effect was observed in the short term with a reduction of type M1 microglia and glutamate levels, but with a subsequent increase of TREM2. In the long term, an improvement of myelination in NG2-IR, an increase in MBP content, and a reduction of the demyelination area were observed. These data demonstrated that NMeds can successfully be used to obtain more controlled local drug delivery and that this multiple treatment could be effective in improving the outcome of SCIs.
Full article
►▼
Show Figures
Open AccessArticle
Identification of Neurocan and Phosphacan as Early Biomarkers for Open Neural Tube Defects
by
Karolina Janik, George M. Smith and Barbara Krynska
Cited by 1 | Viewed by 1373
Abstract
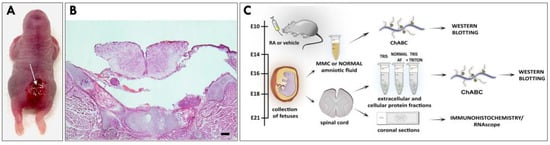
Open neural tube defects (NTDs) such as myelomeningocele (MMC) are debilitating and the most common congenital defects of the central nervous system. Despite their apparent clinical importance, the existing early prenatal diagnostic options for these defects remain limited. Using a well-accepted retinoic-acid-induced model
[...] Read more.
Open neural tube defects (NTDs) such as myelomeningocele (MMC) are debilitating and the most common congenital defects of the central nervous system. Despite their apparent clinical importance, the existing early prenatal diagnostic options for these defects remain limited. Using a well-accepted retinoic-acid-induced model of MMC established in fetal rats, we discovered that neurocan and phosphacan, the secreted chondroitin sulfate proteoglycans of the developing nervous system, are released into the amniotic fluid (AF) of fetal rats displaying spinal cord defects. In contrast to normal controls, elevated AF levels of neurocan and phosphacan were detected in MMC fetuses early in gestation and continued to increase during MMC progression, reaching the highest level in near-term fetuses. The molecular forms of neurocan and phosphacan identified in the AF of MMC fetuses and those found in MMC spinal cords were qualitatively similar. In summary, this is the first report demonstrating the presence of neurocan and phosphacan in the AF of MMC fetuses. The identification of elevated levels of neurocan and phosphacan in the AF of MMC fetuses provides two prospective biomarkers with the potential for early prenatal diagnosis of open NTDs.
Full article
►▼
Show Figures
Open AccessArticle
Intranasal Delivery of miR133b in a NEO100-Based Formulation Induces a Healing Response in Spinal Cord-Injured Mice
by
Camelia A. Danilov, Thu Zan Thein, Stanley M. Tahara, Axel H. Schönthal and Thomas C. Chen
Cited by 1 | Viewed by 1209
Abstract
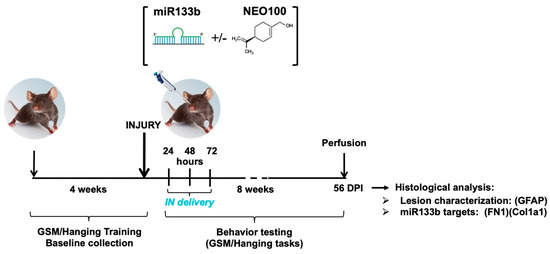
Despite important advances in the pre-clinical animal studies investigating the neuroinhibitory microenvironment at the injury site, traumatic injury to the spinal cord remains a major problem with no concrete response. Here, we examined whether (1) intranasal (IN) administration of miR133b/Ago2 can reach the
[...] Read more.
Despite important advances in the pre-clinical animal studies investigating the neuroinhibitory microenvironment at the injury site, traumatic injury to the spinal cord remains a major problem with no concrete response. Here, we examined whether (1) intranasal (IN) administration of miR133b/Ago2 can reach the injury site and achieve a therapeutic effect and (2) NEO100-based formulation of miR133b/Ago2 can improve effectiveness. 24 h after a cervical contusion, C57BL6 female mice received IN delivery of miR133b/Ago2 or miR133b/Ago2/NEO100 for 3 days, one dose per day. The pharmacokinetics of miR133b in the spinal cord lesion was determined by RT-qPCR. The role of IN delivery of miR133b on motor function was assessed by the grip strength meter (GSM) and hanging tasks. The activity of miR133b at the lesion site was established by immunostaining of fibronectin 1 (FN1), a miR133b target. We found that IN delivery of miR133b/Ago2 (1) reaches the lesion scar and co-administration of miR133b with NEO100 facilitated the cellular uptake; (2) enhanced the motor function and addition of NEO100 potentiated this effect and (3) targeted FN1 expression at the lesion scar. Our results suggest a high efficacy of IN delivery of miR133b/Ago2 to the injured spinal cord that translates to improved healing with NEO100 further potentiating this effect.
Full article
►▼
Show Figures
Open AccessArticle
Composite Fibrin and Carbon Microfibre Implant to Modulate Postraumatic Inflammation after Spinal Cord Injury
by
Vincent Escarrat, Jimena Perez-Sanchez, Bilal El-Waly, Jorge E. Collazos-Castro and Franck Debarbieux
Cited by 1 | Viewed by 1600
Abstract
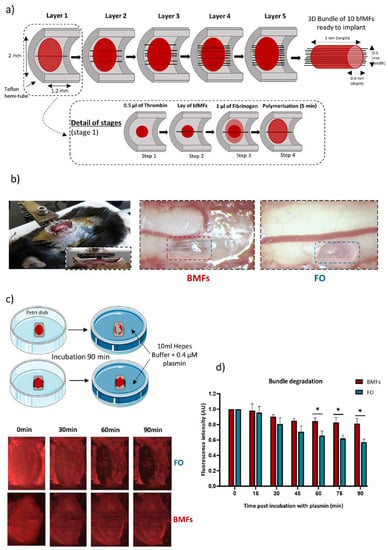
Poor functional recovery after spinal cord injury (SCI) drives the development of novel strategies to manage this devastating condition. We recently showed promising immunomodulatory and pro-regenerative actions of bio-functionalized carbon microfibres (MFs) implanted in a rodent model of SCI. In order to maximize
[...] Read more.
Poor functional recovery after spinal cord injury (SCI) drives the development of novel strategies to manage this devastating condition. We recently showed promising immunomodulatory and pro-regenerative actions of bio-functionalized carbon microfibres (MFs) implanted in a rodent model of SCI. In order to maximize tissue repair while easing MF implantation, we produced a composite implant based on the embedding of several MFs within a fibrin hydrogel. We used intravital imaging of fluorescent reporter mice at the early stages and spinal sections of the same animals 3 months later to characterize the neuroinflammatory response to the implant and its impact on axonal regeneration. Whereas fibrin alone was inert in the first week, its enzymatic degradation drove the chronic activation of microglial cells and axonal degeneration within 3 months. However, the presence of MFs inside the fibrin hydrogel slowed down fibrin degradation and boosted the early recruitment of immune cells. Noteworthy, there was an enhanced contribution of monocyte-derived dendritic cells (moDCs), preceding a faster transition toward an anti-inflammatory environment with increased axonal regeneration over 3 months. The inclusion of MF here ensured the long-term biocompatibility of fibrin hydrogels, which would otherwise preclude successful spinal cord regeneration.
Full article
►▼
Show Figures
Open AccessReview
The Proteostasis Network: A Global Therapeutic Target for Neuroprotection after Spinal Cord Injury
by
Scott R. Whittemore, Sujata Saraswat Ohri, Michael D. Forston, George Z. Wei and Michal Hetman
Cited by 3 | Viewed by 2207
Abstract
Proteostasis (protein homeostasis) is critical for cellular as well as organismal survival. It is strictly regulated by multiple conserved pathways including the ubiquitin-proteasome system, autophagy, the heat shock response, the integrated stress response, and the unfolded protein response. These overlapping proteostasis maintenance modules
[...] Read more.
Proteostasis (protein homeostasis) is critical for cellular as well as organismal survival. It is strictly regulated by multiple conserved pathways including the ubiquitin-proteasome system, autophagy, the heat shock response, the integrated stress response, and the unfolded protein response. These overlapping proteostasis maintenance modules respond to various forms of cellular stress as well as organismal injury. While proteostasis restoration and ultimately organism survival is the main evolutionary driver of such a regulation, unresolved disruption of proteostasis may engage pro-apoptotic mediators of those pathways to eliminate defective cells. In this review, we discuss proteostasis contributions to the pathogenesis of traumatic spinal cord injury (SCI). Most published reports focused on the role of proteostasis networks in acute/sub-acute tissue damage post-SCI. Those reports reveal a complex picture with cell type- and/or proteostasis mediator-specific effects on loss of neurons and/or glia that often translate into the corresponding modulation of functional recovery. Effects of proteostasis networks on such phenomena as neuro-repair, post-injury plasticity, as well as systemic manifestations of SCI including dysregulation of the immune system, metabolism or cardiovascular function are currently understudied. However, as potential interventions that target the proteostasis networks are expected to impact many cell types across multiple organ systems that are compromised after SCI, such therapies could produce beneficial effects across the wide spectrum of highly variable human SCI.
Full article
►▼
Show Figures
Open AccessReview
Ionic Plasticity: Common Mechanistic Underpinnings of Pathology in Spinal Cord Injury and the Brain
by
Kelsey E. Hudson and James W. Grau
Cited by 4 | Viewed by 2560
Abstract
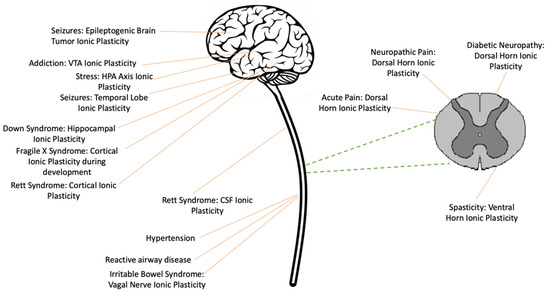
The neurotransmitter GABA is normally characterized as having an inhibitory effect on neural activity in the adult central nervous system (CNS), which quells over-excitation and limits neural plasticity. Spinal cord injury (SCI) can bring about a modification that weakens the inhibitory effect of
[...] Read more.
The neurotransmitter GABA is normally characterized as having an inhibitory effect on neural activity in the adult central nervous system (CNS), which quells over-excitation and limits neural plasticity. Spinal cord injury (SCI) can bring about a modification that weakens the inhibitory effect of GABA in the central gray caudal to injury. This change is linked to the downregulation of the potassium/chloride cotransporter (KCC2) and the consequent rise in intracellular Cl
− in the postsynaptic neuron. As the intracellular concentration increases, the inward flow of Cl
− through an ionotropic GABA-A receptor is reduced, which decreases its hyperpolarizing (inhibitory) effect, a modulatory effect known as ionic plasticity. The loss of GABA-dependent inhibition enables a state of over-excitation within the spinal cord that fosters aberrant motor activity (spasticity) and chronic pain. A downregulation of KCC2 also contributes to the development of a number of brain-dependent pathologies linked to states of neural over-excitation, including epilepsy, addiction, and developmental disorders, along with other diseases such as hypertension, asthma, and irritable bowel syndrome. Pharmacological treatments that target ionic plasticity have been shown to bring therapeutic benefits.
Full article
►▼
Show Figures
Open AccessReview
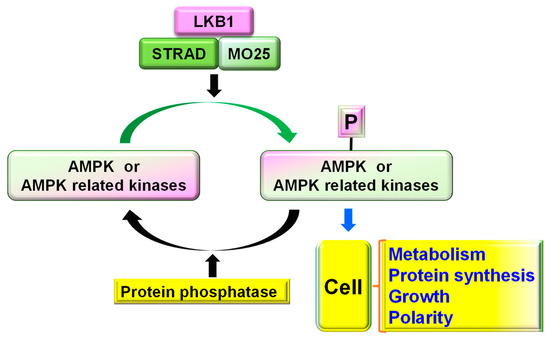
Liver Kinase B1 Functions as a Regulator for Neural Development and a Therapeutic Target for Neural Repair
by
En Huang and Shuxin Li
Cited by 3 | Viewed by 2082
Abstract
The liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11) and Par-4 in
C. elegans, has been identified as a master kinase of AMPKs and AMPK-related kinases. LKB1 plays a crucial role in cell growth, metabolism, polarity, and tumor suppression.
[...] Read more.
The liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11) and Par-4 in
C. elegans, has been identified as a master kinase of AMPKs and AMPK-related kinases. LKB1 plays a crucial role in cell growth, metabolism, polarity, and tumor suppression. By interacting with the downstream signals of SAD, NUAK, MARK, and other kinases, LKB1 is critical to regulating neuronal polarization and axon branching during development. It also regulates Schwann cell function and the myelination of peripheral axons. Regulating LKB1 activity has become an attractive strategy for repairing an injured nervous system. LKB1 upregulation enhances the regenerative capacity of adult CNS neurons and the recovery of locomotor function in adult rodents with CNS axon injury. Here, we update the major cellular and molecular mechanisms of LKB1 in regulating neuronal polarization and neural development, and the implications thereof for promoting neural repair, axon regeneration, and functional recovery in adult mammals.
Full article
►▼
Show Figures
Open AccessArticle
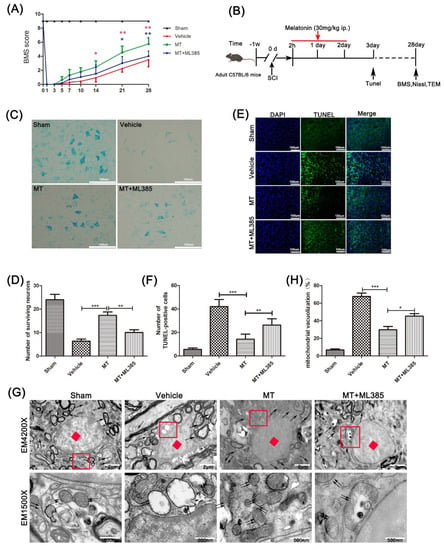
Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome
by
Haoyu Wang, Haifan Wang, Heng Huang, Zhigang Qu, Dong Ma, Xiaoqian Dang and Quanyu Dong
Cited by 12 | Viewed by 2363
Abstract
Background: Spinal cord injury (SCI) is a central nervous system (CNS) trauma involving inflammation and oxidative stress, which play important roles in this trauma’s pathogenesis. Therefore, controlling inflammation is an effective strategy for SCI treatment. As a hormone, melatonin is capable of producing
[...] Read more.
Background: Spinal cord injury (SCI) is a central nervous system (CNS) trauma involving inflammation and oxidative stress, which play important roles in this trauma’s pathogenesis. Therefore, controlling inflammation is an effective strategy for SCI treatment. As a hormone, melatonin is capable of producing antioxidation and anti-inflammation effects. In the meantime, it also causes a neuroprotective effect in various neurological diseases. Nrf2/ARE/NLRP3 is a well-known pathway in anti-inflammation and antioxidation, and Nrf2 can be positively regulated by melatonin. However, how melatonin regulates inflammation during SCI is poorly explored. Therefore, it was investigated in this study whether melatonin can inhibit the NLRP3 inflammasome through the Nrf2/ARE signaling pathway in a mouse SCI model. Methods: A model of SCI was established in C57BL/6 mice and PC12 cells. The motor function of mice was detected by performing an open field test, and Nissl staining and terminal deoxynucleotidyl transferase dUTP nick end labeling were carried out to evaluate the survival of neurons. Mitochondrial dysfunction was detected by transmission electron microscopy (TEM) and by assessing the mitochondrial membrane potential. In addition, the expression of NLRP3 inflammasome and oxidative-stress-related proteins were detected through Western blot and immunofluorescence double staining. Results: By inhibiting neuroinflammation and reducing neuronal death, melatonin promotes the recovery of neuromotor function. Besides this, melatonin is able to reduce the damage that causes neuronal mitochondrial dysfunction, reduce the level of reactive oxygen species (ROS) and malondialdehyde, and enhance the activity of superoxide dismutase and the production of glutathione peroxidase. Mechanically, melatonin inhibits the activation of NLRP3 inflammasomes and reduces the secretion of pro-inflammatory factors through the Nrf2/ARE signaling. Conclusions: In conclusion, melatonin inhibits the NLRP3 inflammasome through stimulation of the Nrf2/ARE pathway, thereby suppressing neuroinflammation, reducing mitochondrial dysfunction, and improving the recovery of nerve function after SCI.
Full article
►▼
Show Figures
Open AccessArticle
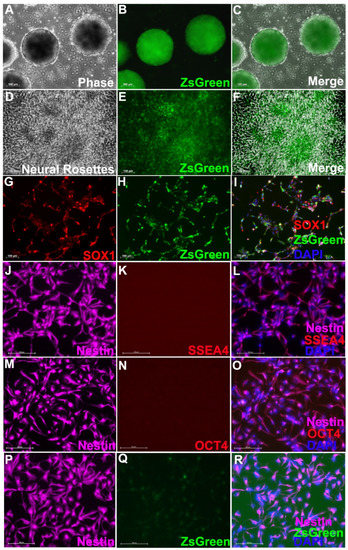
Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury
by
Yiyan Zheng, Chrystine M. Gallegos, Haipeng Xue, Shenglan Li, Dong H. Kim, Hongxia Zhou, Xugang Xia, Ying Liu and Qilin Cao
Cited by 4 | Viewed by 2091
Abstract
Locomotor function after spinal cord injury (SCI) is critical for assessing recovery. Currently, available means to improve locomotor function include surgery, physical therapy rehabilitation and exoskeleton. Stem cell therapy with neural progenitor cells (NPCs) transplantation is a promising reparative strategy. Along this line,
[...] Read more.
Locomotor function after spinal cord injury (SCI) is critical for assessing recovery. Currently, available means to improve locomotor function include surgery, physical therapy rehabilitation and exoskeleton. Stem cell therapy with neural progenitor cells (NPCs) transplantation is a promising reparative strategy. Along this line, patient-specific induced pluripotent stem cells (iPSCs) are a remarkable autologous cell source, which offer many advantages including: great potential to generate isografts avoiding immunosuppression; the availability of a variety of somatic cells without ethical controversy related to embryo use; and vast differentiation. In this current work, to realize the therapeutic potential of iPSC-NPCs for the treatment of SCI, we transplanted purified iPSCs-derived NPCs into a cervical contusion SCI rat model. Our results showed that the iPSC-NPCs were able to survive and differentiate into both neurons and astrocytes and, importantly, improve forelimb locomotor function as assessed by the grooming task and horizontal ladder test. Purified iPSC-NPCs represent a promising cell type that could be further tested and developed into a clinically useful cell source for targeted cell therapy for cervical SCI patients.
Full article
►▼
Show Figures
Open AccessReview
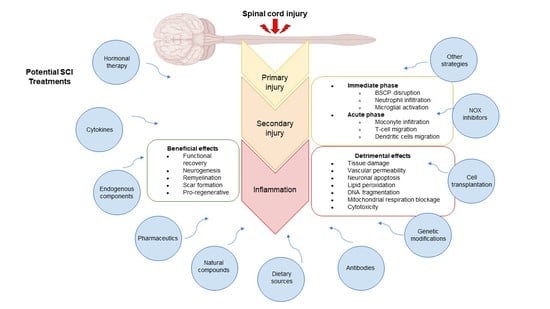
Inflammation: A Target for Treatment in Spinal Cord Injury
by
Ximena Freyermuth-Trujillo, Julia J. Segura-Uribe, Hermelinda Salgado-Ceballos, Carlos E. Orozco-Barrios and Angélica Coyoy-Salgado
Cited by 30 | Viewed by 3698
Abstract
Spinal cord injury (SCI) is a significant cause of disability, and treatment alternatives that generate beneficial outcomes and have no side effects are urgently needed. SCI may be treatable if intervention is initiated promptly. Therefore, several treatment proposals are currently being evaluated. Inflammation
[...] Read more.
Spinal cord injury (SCI) is a significant cause of disability, and treatment alternatives that generate beneficial outcomes and have no side effects are urgently needed. SCI may be treatable if intervention is initiated promptly. Therefore, several treatment proposals are currently being evaluated. Inflammation is part of a complex physiological response to injury or harmful stimuli induced by mechanical, chemical, or immunological agents. Neuroinflammation is one of the principal secondary changes following SCI and plays a crucial role in modulating the pathological progression of acute and chronic SCI. This review describes the main inflammatory events occurring after SCI and discusses recently proposed potential treatments and therapeutic agents that regulate inflammation after insult in animal models.
Full article
►▼
Show Figures
Open AccessSystematic Review
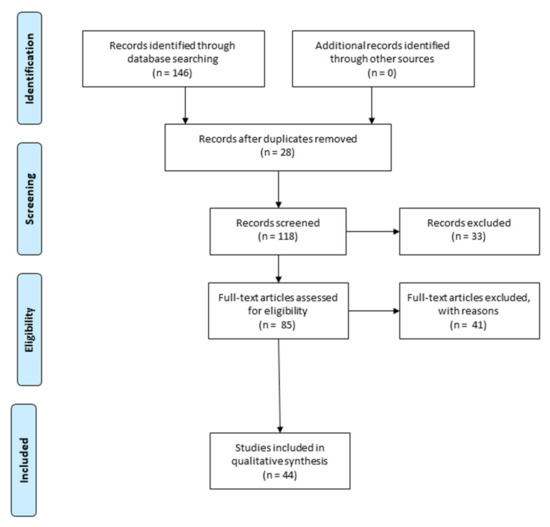
Main Cations and Cellular Biology of Traumatic Spinal Cord Injury
by
Constantin Munteanu, Mariana Rotariu, Marius Turnea, Anca Mirela Ionescu, Cristina Popescu, Aura Spinu, Elena Valentina Ionescu, Carmen Oprea, Roxana Elena Țucmeanu, Ligia Gabriela Tătăranu, Sînziana Calina Silișteanu and Gelu Onose
Cited by 8 | Viewed by 2973
Abstract
Traumatic spinal cord injury is a life-changing condition with a significant socio-economic impact on patients, their relatives, their caregivers, and even the community. Despite considerable medical advances, there is still a lack of options for the effective treatment of these patients. The major
[...] Read more.
Traumatic spinal cord injury is a life-changing condition with a significant socio-economic impact on patients, their relatives, their caregivers, and even the community. Despite considerable medical advances, there is still a lack of options for the effective treatment of these patients. The major complexity and significant disabling potential of the pathophysiology that spinal cord trauma triggers are the main factors that have led to incremental scientific research on this topic, including trying to describe the molecular and cellular mechanisms that regulate spinal cord repair and regeneration. Scientists have identified various practical approaches to promote cell growth and survival, remyelination, and neuroplasticity in this part of the central nervous system. This review focuses on specific detailed aspects of the involvement of cations in the cell biology of such pathology and on the possibility of repairing damaged spinal cord tissue. In this context, the cellular biology of sodium, potassium, lithium, calcium, and magnesium is essential for understanding the related pathophysiology and also the possibilities to counteract the harmful effects of traumatic events. Lithium, sodium, potassium—monovalent cations—and calcium and magnesium—bivalent cations—can influence many protein–protein interactions, gene transcription, ion channel functions, cellular energy processes—phosphorylation, oxidation—inflammation, etc. For data systematization and synthesis, we used the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) methodology, trying to make, as far as possible, some order in seeing the “big forest” instead of “trees”. Although we would have expected a large number of articles to address the topic, we were still surprised to find only 51 unique articles after removing duplicates from the 207 articles initially identified. Our article integrates data on many biochemical processes influenced by cations at the molecular level to understand the real possibilities of therapeutic intervention—which must maintain a very narrow balance in cell ion concentrations. Multimolecular, multi-cellular: neuronal cells, glial cells, non-neuronal cells, but also multi-ionic interactions play an important role in the balance between neuro-degenerative pathophysiological processes and the development of effective neuroprotective strategies. This article emphasizes the need for studying cation dynamics as an important future direction.
Full article
►▼
Show Figures
Open AccessReview
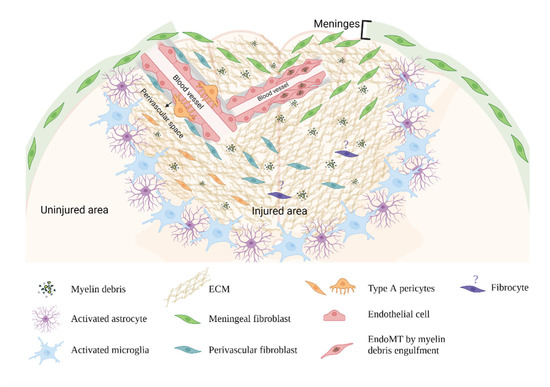
Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation
by
Maryam Ayazi, Sandra Zivkovic, Grace Hammel, Branko Stefanovic and Yi Ren
Cited by 14 | Viewed by 3650
Abstract
Central nervous system (CNS) trauma activates a persistent repair response that leads to fibrotic scar formation within the lesion. This scarring is similar to other organ fibrosis in many ways; however, the unique features of the CNS differentiate it from other organs. In
[...] Read more.
Central nervous system (CNS) trauma activates a persistent repair response that leads to fibrotic scar formation within the lesion. This scarring is similar to other organ fibrosis in many ways; however, the unique features of the CNS differentiate it from other organs. In this review, we discuss fibrotic scar formation in CNS trauma, including the cellular origins of fibroblasts, the mechanism of fibrotic scar formation following an injury, as well as the implication of the fibrotic scar in CNS tissue remodeling and regeneration. While discussing the shared features of CNS fibrotic scar and fibrosis outside the CNS, we highlight their differences and discuss therapeutic targets that may enhance regeneration in the CNS.
Full article
►▼
Show Figures
Open AccessArticle
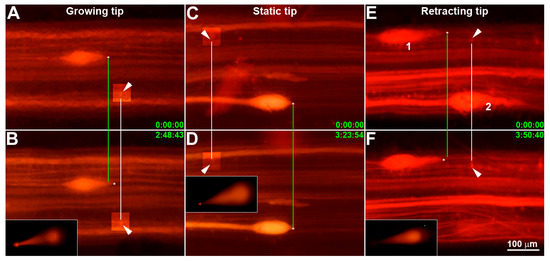
Transcriptomes of Injured Lamprey Axon Tips: Single-Cell RNA-Seq Suggests Differential Involvement of MAPK Signaling Pathways in Axon Retraction and Regeneration after Spinal Cord Injury
by
Li-Qing Jin, Yan Zhou, Yue-Sheng Li, Guixin Zhang, Jianli Hu and Michael E. Selzer
Cited by 4 | Viewed by 2497
Abstract
Axotomy in the CNS activates retrograde signals that can trigger regeneration or cell death. Whether these outcomes use different injury signals is not known. Local protein synthesis in axon tips plays an important role in axon retraction and regeneration. Microarray and RNA-seq studies
[...] Read more.
Axotomy in the CNS activates retrograde signals that can trigger regeneration or cell death. Whether these outcomes use different injury signals is not known. Local protein synthesis in axon tips plays an important role in axon retraction and regeneration. Microarray and RNA-seq studies on cultured mammalian embryonic or early postnatal peripheral neurons showed that axon growth cones contain hundreds to thousands of mRNAs. In the lamprey, identified reticulospinal neurons vary in the probability that their axons will regenerate after axotomy. The bad regenerators undergo early severe axon retraction and very delayed apoptosis. We micro-aspirated axoplasms from 10 growing, 9 static and 5 retracting axon tips of spinal cord transected lampreys and performed single-cell RNA-seq, analyzing the results bioinformatically. Genes were identified that were upregulated selectively in growing (
n = 38), static (20) or retracting tips (18). Among them,
map3k2,
csnk1e and
gtf2h were expressed in growing tips,
mapk8(1) was expressed in static tips and
prkcq was expressed in retracting tips. Venn diagrams revealed more than 40 components of MAPK signaling pathways, including
jnk and
p38 isoforms, which were differentially distributed in growing, static and/or retracting tips. Real-time q-PCR and immunohistochemistry verified the colocalization of
map3k2 and
csnk1e in growing axon tips. Thus, differentially regulated MAPK and circadian rhythm signaling pathways may be involved in activating either programs for axon regeneration or axon retraction and apoptosis.
Full article
►▼
Show Figures
Open AccessArticle
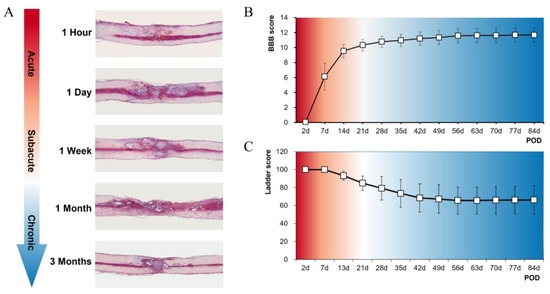
The Time Sequence of Gene Expression Changes after Spinal Cord Injury
by
Seyoung Mun, Kyudong Han and Jung Keun Hyun
Cited by 2 | Viewed by 2429
Abstract
Gene expression changes following spinal cord injury (SCI) are time-dependent, and an accurate understanding of these changes can be crucial in determining time-based treatment options in a clinical setting. We performed RNA sequencing of the contused spinal cord of rats at five different
[...] Read more.
Gene expression changes following spinal cord injury (SCI) are time-dependent, and an accurate understanding of these changes can be crucial in determining time-based treatment options in a clinical setting. We performed RNA sequencing of the contused spinal cord of rats at five different time points from the very acute to chronic stages (1 hour, 1 day, 1 week, 1 month, and 3 months) following SCI. We identified differentially expressed genes (DEGs) and Gene Ontology (GO) terms at each time point, and 14,257 genes were commonly expressed at all time points. The biological process of the inflammatory response was increased at 1 hour and 1 day, and the cellular component of the integral component of the synaptic membrane was increased at 1 day. DEGs associated with cell activation and the innate immune response were highly enriched at 1 week and 1 month, respectively. A total of 2841 DEGs were differentially expressed at any of the five time points, and 18 genes (17 upregulated and 1 downregulated) showed common expression differences at all time points. We found that interleukin signaling, neutrophil degranulation, eukaryotic translation, collagen degradation, LGI–ADAM interactions, GABA receptor, and L1CAM-ankyrin interactions were prominent after SCI depending on the time post injury. We also performed gene–drug network analysis and found several potential antagonists and agonists which can be used to treat SCI. We expect to discover effective treatments in the clinical field through further studies revealing the efficacy and safety of potential drugs.
Full article
►▼
Show Figures
Open AccessReview
MiRNAs as Promising Translational Strategies for Neuronal Repair and Regeneration in Spinal Cord Injury
by
Serena Silvestro and Emanuela Mazzon
Cited by 12 | Viewed by 2743
Abstract
Spinal cord injury (SCI) represents a devastating injury to the central nervous system (CNS) that is responsible for impaired mobility and sensory function in SCI patients. The hallmarks of SCI include neuroinflammation, axonal degeneration, neuronal loss, and reactive gliosis. Current strategies, including stem
[...] Read more.
Spinal cord injury (SCI) represents a devastating injury to the central nervous system (CNS) that is responsible for impaired mobility and sensory function in SCI patients. The hallmarks of SCI include neuroinflammation, axonal degeneration, neuronal loss, and reactive gliosis. Current strategies, including stem cell transplantation, have not led to successful clinical therapy. MiRNAs are crucial for the differentiation of neural cell types during CNS development, as well as for pathological processes after neural injury including SCI. This makes them ideal candidates for therapy in this condition. Indeed, several studies have demonstrated the involvement of miRNAs that are expressed differently in CNS injury. In this context, the purpose of the review is to provide an overview of the pre-clinical evidence evaluating the use of miRNA therapy in SCI. Specifically, we have focused our attention on miRNAs that are widely associated with neuronal and axon regeneration. “MiRNA replacement therapy” aims to transfer miRNAs to diseased cells and improve targeting efficacy in the cells, and this new therapeutic tool could provide a promising technique to promote SCI repair and reduce functional deficits.
Full article
►▼
Show Figures
Open AccessArticle
Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton’s Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance
by
Joaquim Vives, Joaquim Hernández, Clémentine Mirabel, Maria Puigdomenech-Poch, David Romeo-Guitart, Sara Marmolejo-Martínez-Artesero, Raquel Cabrera-Pérez, Jessica Jaramillo, Hatice Kumru, Joan García-López, Joan Vidal-Samsó, Xavier Navarro and Ruth Coll-Bonet
Cited by 1 | Viewed by 2320
Abstract
(1) Background: the use of Mesenchymal Stromal Cells (MSC) in emerging therapies for spinal cord injury (SCI) hold the potential to improve functional recovery. However, the development of cell-based medicines is challenging and preclinical studies addressing quality, safety and efficacy must be conducted
[...] Read more.
(1) Background: the use of Mesenchymal Stromal Cells (MSC) in emerging therapies for spinal cord injury (SCI) hold the potential to improve functional recovery. However, the development of cell-based medicines is challenging and preclinical studies addressing quality, safety and efficacy must be conducted prior to clinical testing; (2) Methods: herein we present (i) the characterization of the quality attributes of MSC from the Wharton’s jelly (WJ) of the umbilical cord, (ii) safety of intrathecal infusion in a 3-month subchronic toxicity assessment study, and (iii) efficacy in a rat SCI model by controlled impaction (100 kdynes) after single (day 7 post-injury) and repeated dose of 1 × 10
6 MSC,WJ (days 7 and 14 post-injury) with 70-day monitoring by electrophysiological testing, motor function assessment and histology evaluation; (3) Results: no toxicity associated to MSC,WJ infusion was observed. Regarding efficacy, recovery of locomotion was promoted at early time points. Persistence of MSC,WJ was detected early after administration (day 2 post-injection) but not at days 14 and 63 post-injection. (4) Conclusions: the safety profile and signs of efficacy substantiate the suitability of the presented data for inclusion in the Investigational Medicinal Product Dossier for further consideration by the competent Regulatory Authority to proceed with clinical trials.
Full article
►▼
Show Figures
Open AccessArticle
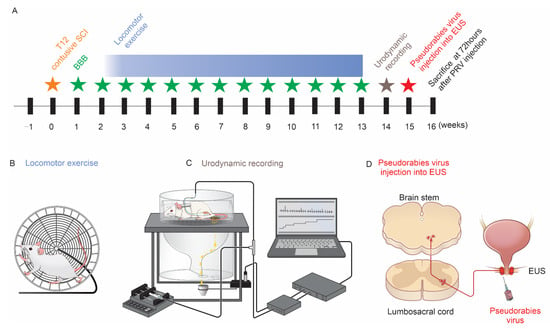
Locomotor Exercise Enhances Supraspinal Control of Lower-Urinary-Tract Activity to Improve Micturition Function after Contusive Spinal-Cord Injury
by
Lingxiao Deng, Tao Sui, Dong V. Wang, Shaoping Hou, Xiaojian Cao, Kaiwen Peng, Zaocheng Xu and Xiaoming Xu
Cited by 1 | Viewed by 2355
Abstract
The recovery of lower-urinary-tract activity is a top priority for patients with spinal-cord injury. Historically, locomotor training improved micturition function in both patients with spinal cord injury and animal models. We explore whether training augments such as the supraspinal control of the external
[...] Read more.
The recovery of lower-urinary-tract activity is a top priority for patients with spinal-cord injury. Historically, locomotor training improved micturition function in both patients with spinal cord injury and animal models. We explore whether training augments such as the supraspinal control of the external urethral sphincter results in enhanced coordination in detrusor-sphincter activity. We implemented a clinically relevant contusive spinal-cord injury at the 12th thoracic level in rats and administered forced wheel running exercise for 11 weeks. Awake rats then underwent bladder cystometrogram and sphincter electromyography recordings to examine the micturition reflex. Subsequently, pseudorabies-virus-encoding red fluorescent protein was injected into the sphincter to trans-synaptically trace the supraspinal innervation of Onuf’s motoneurons. Training in the injury group reduced the occurrence of bladder nonvoiding contractions, decreased the voiding threshold and peak intravesical pressure, and shortened the latency of sphincter bursting during voiding, leading to enhanced voiding efficiency. Histological analysis demonstrated that the training increased the extent of spared spinal-cord tissue around the epicenter of lesions. Compared to the group of injury without exercise, training elicited denser 5-hydroxytryptamine-positive axon terminals in the vicinity of Onuf’s motoneurons in the cord; more pseudorabies virus-labeled or c-fos expressing neurons were detected in the brainstem, suggesting the enhanced supraspinal control of sphincter activity. Thus, locomotor training promotes tissue sparing and axon innervation of spinal motoneurons to improve voiding function following contusive spinal-cord injury.
Full article
►▼
Show Figures
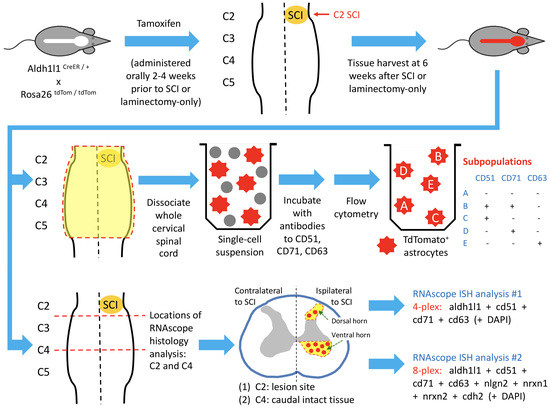
Open AccessEditor’s ChoiceArticle
Response of Astrocyte Subpopulations Following Spinal Cord Injury
by
R. Vivian Allahyari, Nicolette M. Heinsinger, Daniel Hwang, David A. Jaffe, Javad Rasouli, Stephanie Shiers, Samantha J. Thomas, Theodore J. Price, Abdolmohamad Rostami and Angelo C. Lepore
Cited by 8 | Viewed by 3263
Abstract
There is growing appreciation for astrocyte heterogeneity both across and within central nervous system (CNS) regions, as well as between intact and diseased states. Recent work identified multiple astrocyte subpopulations in mature brain. Interestingly, one subpopulation (Population C) was shown to possess significantly
[...] Read more.
There is growing appreciation for astrocyte heterogeneity both across and within central nervous system (CNS) regions, as well as between intact and diseased states. Recent work identified multiple astrocyte subpopulations in mature brain. Interestingly, one subpopulation (Population C) was shown to possess significantly enhanced synaptogenic properties in vitro, as compared with other astrocyte subpopulations of adult cortex and spinal cord. Following spinal cord injury (SCI), damaged neurons lose synaptic connections with neuronal partners, resulting in persistent functional loss. We determined whether SCI induces an enhanced synaptomodulatory astrocyte phenotype by shifting toward a greater proportion of Population C cells and/or increasing expression of relevant synapse formation-associated genes within one or more astrocyte subpopulations. Using flow cytometry and RNAscope in situ hybridization, we found that astrocyte subpopulation distribution in the spinal cord did not change to a selectively synaptogenic phenotype following mouse cervical hemisection-type SCI. We also found that spinal cord astrocytes expressed synapse formation-associated genes to a similar degree across subpopulations, as well as in an unchanged manner between uninjured and SCI conditions. Finally, we confirmed these astrocyte subpopulations are also present in the human spinal cord in a similar distribution as mouse, suggesting possible conservation of spinal cord astrocyte heterogeneity across species.
Full article
►▼
Show Figures
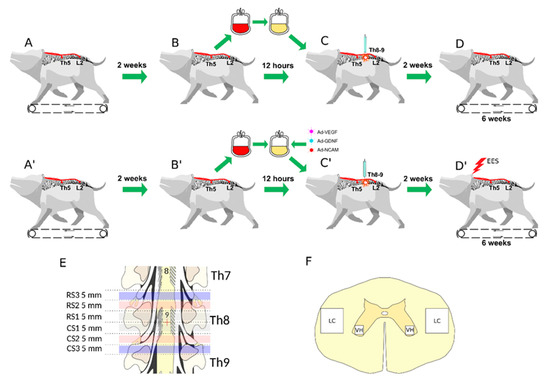
Open AccessArticle
New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation
by
Rustem Islamov, Farid Bashirov, Andrei Izmailov, Filip Fadeev, Vage Markosyan, Mikhail Sokolov, Maksim Shmarov, Denis Logunov, Boris Naroditsky and Igor Lavrov
Cited by 8 | Viewed by 2673
Abstract
The contemporary strategy for spinal cord injury (SCI) therapy aims to combine multiple approaches to control pathogenic mechanisms of neurodegeneration and stimulate neuroregeneration. In this study, a novel regenerative approach using an autologous leucoconcentrate enriched with transgenes encoding vascular endothelial growth factor (VEGF),
[...] Read more.
The contemporary strategy for spinal cord injury (SCI) therapy aims to combine multiple approaches to control pathogenic mechanisms of neurodegeneration and stimulate neuroregeneration. In this study, a novel regenerative approach using an autologous leucoconcentrate enriched with transgenes encoding vascular endothelial growth factor (VEGF), glial cell line-derived neurotrophic factor (GDNF), and neural cell adhesion molecule (NCAM) combined with supra- and sub-lesional epidural electrical stimulation (EES) was tested on mini-pigs similar in morpho-physiological scale to humans. The complex analysis of the spinal cord recovery after a moderate contusion injury in treated mini-pigs compared to control animals revealed: better performance in behavioural and joint kinematics, restoration of electromyography characteristics, and improvement in selected immunohistology features related to cell survivability, synaptic protein expression, and glial reorganization above and below the injury. These results for the first time demonstrate the positive effect of intravenous infusion of autologous genetically-enriched leucoconcentrate producing recombinant molecules stimulating neuroregeneration combined with neuromodulation by translesional multisite EES on the restoration of the post-traumatic spinal cord in mini-pigs and suggest the high translational potential of this novel regenerative therapy for SCI patients.
Full article
►▼
Show Figures
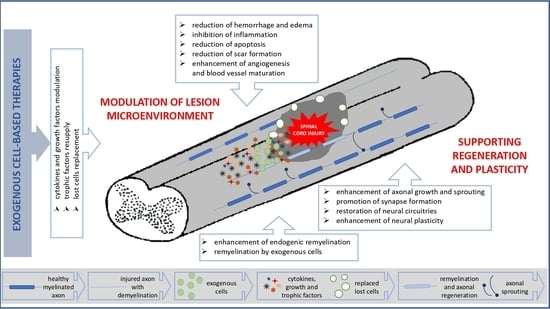
Open AccessReview
Perspectives in the Cell-Based Therapies of Various Aspects of the Spinal Cord Injury-Associated Pathologies: Lessons from the Animal Models
by
Małgorzata Zawadzka, Anna Kwaśniewska, Krzysztof Miazga and Urszula Sławińska
Cited by 12 | Viewed by 2608
Abstract
Traumatic injury of the spinal cord (SCI) is a devastating neurological condition often leading to severe dysfunctions, therefore an improvement in clinical treatment for SCI patients is urgently needed. The potential benefits of transplantation of various cell types into the injured spinal cord
[...] Read more.
Traumatic injury of the spinal cord (SCI) is a devastating neurological condition often leading to severe dysfunctions, therefore an improvement in clinical treatment for SCI patients is urgently needed. The potential benefits of transplantation of various cell types into the injured spinal cord have been intensively investigated in preclinical SCI models and clinical trials. Despite the many challenges that are still ahead, cell transplantation alone or in combination with other factors, such as artificial matrices, seems to be the most promising perspective. Here, we reviewed recent advances in cell-based experimental strategies supporting or restoring the function of the injured spinal cord with a particular focus on the regenerative mechanisms that could define their clinical translation.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Spinal Cord Injury Pain: Mechanisms and Potential Therapies
Authors: Tetyana Martynyuk; Megan Detloff; John Bethea
Affiliation: Tetyana Martynyuk and Dr. Bethea- Drexel University, Department of Biology and Dr. Detloff is Drexel University, College of Medicine, Department of Neurobiology & Anatomy
Abstract: /
Title: Ionic plasticity: common mechanistic underpinnings of pathology in spinal cord injury and the brain
Authors: Jim Grau; Kelsey Hudson
Affiliation: Neuroscience and the Department of Psychological and Brain Sciences, Texas A&M University, College Station TX
Abstract: /
Title: Involvement of cations in the cellular biology of traumatic Spinal Cord Injury (SCI) and Repair
Authors: Constantin Munteanu; Marius Turnea; Sînziana Călina Șilișteanu; Mariana Rotariu
Affiliation: University of Medicine and Pharmacy „Gr. T. Popa ”, Iași; University ”Ștefan cel Mare, Suceava
Abstract: /
Title: Melatonin attenuates spinal cord injury in mice by inhibiting the activation of NLRP3 inflammasome to activating Nrf2 / ARE signaling pathway
Authors: Haoyu Wang; Haifan Wang; Heng Huang; Zhigang Qu; Dong Ma; Quanyu Dong
Affiliation: /
Abstract: /
Title: Fibrotic scar in CNS injuries: current knowledge to future direction
Authors: Maryam Ayazi; Sandra Zivkovic; Grace Hammel; Yi Ren
Affiliation: Department of Biomedical Sciences, Florida State University College of Medicine, Tallahassee, FL, 32306, USA
Abstract: /