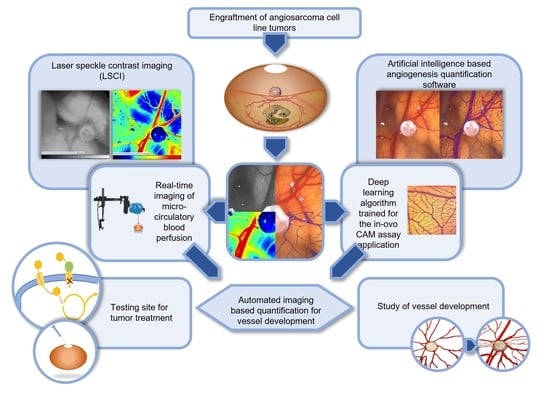
Deep Learning-Based Image Analysis for the Quantification of Tumor-Induced Angiogenesis in the 3D In Vivo Tumor Model—Establishment and Addition to Laser Speckle Contrast Imaging (LSCI)
Abstract
:1. Introduction
1.1. Tumor Angiogenesis and the CAM Model
1.2. Previous Measurement Methods of Angiogenesis in the CAM Model
1.3. Novel Measurement Method for the Quantification of Angiogenesis in the CAM Model
1.4. LSCI as an Established Second Independent Angiogenesis Measurement Method
1.5. Testing of Anti-Angiogenic Agents on Angiosarcomas in the CAM Model
2. Materials and Methods
2.1. CAM Model
2.2. Engraftment of AS-M Cells on the CAM
2.3. Training and Establishment of the CAM Assay Application for the Application in the in ovo CAM Model
2.4. First Independent Angiogenesis Measurement Technique: The CAM Assay Application of the IKOSA Platform
2.5. Second Independent Angiogenesis Measurement Technique: Laser Speckle Contrast Imaging (LSCI)
2.6. Histological Analysis
2.7. Treatment with Sodium Gluconate
2.8. Statistical Methods
3. Results
3.1. Growth of Angiosarcoma Cell Line Tumors on the CAM
3.2. Establishment and Improvement of the CAM Assay Application with Deep Learning
3.3. Analysis of the Tumor Induced Angiogenesis via LSCI
3.4. Assessment of Angiogenesis after Treatment with Gluconate Using LSCI and the CAM Assay Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Ausprunk, D.H.; Folkman, J. Vascular injury in transplanted tissues. Fine structural changes in tumor, adult, and embryonic blood vessels. Virchows Arch. B Cell Pathol. 1976, 21, 31–44. [Google Scholar] [CrossRef]
- Peyton Rous, M.D.; James, B.; Murphy, M.D. Tumor implantations in the developing embryo. JAMA 1911, LVI, 741–742. [Google Scholar] [CrossRef]
- Taylor, A.; Williams, R.J. The control of a cancer growth in embryonated eggs. Proc. Natl. Acad. Sci. USA 1956, 42, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, D.A.; Langer, R.S.; Pless, N.A.; Folkman, J. Mast cells and tumor angiogenesis. Int. J. Cancer 1976, 18, 703–709. [Google Scholar] [CrossRef]
- Cao, J.; Liu, X.; Yang, Y.; Wei, B.; Li, Q.; Mao, G.; He, Y.; Li, Y.; Zheng, L.; Zhang, Q.; et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/ BAI1 signaling pathway. Angiogenesis 2020, 23, 325–338. [Google Scholar] [CrossRef]
- Sys, G.; van Bockstal, M.; Forsyth, R.; Balke, M.; Poffyn, B.; Uyttendaele, D.; Bracke, M.; de Wever, O. Tumor grafts derived from sarcoma patients retain tumor morphology, viability, and invasion potential and indicate disease outcomes in the chick chorioallantoic membrane model. Cancer Lett. 2012, 326, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The first evidence of the tumor-induced angiogenesis in vivo by using the chorioallantoic membrane assay dated 1913. Leukemia 2004, 18, 1350–1351. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [Green Version]
- Valdes, T.I.; Kreutzer, D.; Moussy, F. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J. Biomed. Mater. Res. 2002, 62, 273–282. [Google Scholar] [CrossRef]
- Troebs, J.; Asam, C.; Pion, E.; Prantl, L.; Aung, T.; Haerteis, S. 3D monitoring of tumor volume in an in vivo model. Clin. Hemorheol. Microcirc. 2020, 76, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Feder, A.-L.; Härteis, S. Angiosarkome-eine heterogene vaskuläre Neoplasie mit variabler klinischer Präsentation. J. Onkol. 2020, 20, 33–40. [Google Scholar]
- Kohl, C.; Aung, T.; Haerteis, S.; Ignatov, A.; Ortmann, O.; Papathemelis, T. The 3D in vivo chorioallantoic membrane model and its role in breast cancer research. J. Cancer Res. Clin. Oncol. 2022, 148, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Feder, A.-L.; Pion, E.; Troebs, J.; Lenze, U.; Prantl, L.; Htwe, M.M.; Phyo, A.; Haerteis, S.; Aung, T. Extended analysis of intratumoral heterogeneity of primary osteosarcoma tissue using 3D-in-vivo-tumor-model. Clin. Hemorheol. Microcirc. 2020, 76, 133–141. [Google Scholar] [CrossRef]
- Sperling, S.; Aung, T.; Martin, S.; Rohde, V.; Ninkovic, M. Riluzole: A potential therapeutic intervention in human brain tumor stem-like cells. Oncotarget 2017, 8, 96697–96709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, P.; Schenker, A.; Sähr, H.; Lehner, B.; Fellenberg, J. Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma. PLoS ONE 2019, 14, e0215312. [Google Scholar] [CrossRef]
- Schneiderhan, W.; Diaz, F.; Fundel, M.; Zhou, S.; Siech, M.; Hasel, C.; Möller, P.; Gschwend, J.E.; Seufferlein, T.; Gress, T.; et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J. Cell Sci. 2007, 120, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wang, J.; He, C.; Fang, M. Angiosarcoma: A review of diagnosis and current treatment. Am. J. Cancer Res. 2019, 9, 2303–2313. [Google Scholar]
- Buehler, D.; Rice, S.R.; Moody, J.S.; Rush, P.; Hafez, G.-R.; Attia, S.; Longley, B.J.; Kozak, K.R. Angiosarcoma outcomes and prognostic factors: A 25-year single institution experience. Am. J. Clin. Oncol. 2014, 37, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataramani, V.; Küffer, S.; Cheung, K.C.P.; Jiang, X.; Trümper, L.; Wulf, G.G.; Ströbel, P. CD31 Expression Determines Redox Status and Chemoresistance in Human Angiosarcomas. Clin. Cancer Res. 2018, 24, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalvo, J.; Spencer, C.; Hackathorn, A.; Masterjohn, K.; Perkins, A.; Doty, C.; Arumugam, A.; Ongusaha, P.P.; Lakshmanaswamy, R.; Liao, J.K.; et al. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr. Mol. Med. 2013, 13, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.M.; Amaya, C.; Rains, S.; Diaz, D.; Pham, R.; Battiste, J.; Modiano, J.F.; Kokta, V.; Boucheron, L.E.; Mitchell, D.C.; et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS ONE 2013, 8, e60021. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangır, N.; Hillary, C.J.; Chapple, C.R.; MacNeil, S. Oestradiol-releasing Biodegradable Mesh Stimulates Collagen Production and Angiogenesis: An Approach to Improving Biomaterial Integration in Pelvic Floor Repair. Eur. Urol. Focus 2019, 5, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [Green Version]
- Mangir, N.; Dikici, S.; Claeyssens, F.; MacNeil, S. Using ex Ovo Chick Chorioallantoic Membrane (CAM) Assay To Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.M.; Păunescu, E.; Nowak-Sliwinska, P.; Griffioen, A.W.; Scopelliti, R.; Dyson, P.J. Discovery of a highly tumor-selective organometallic ruthenium(II)-arene complex. J. Med. Chem. 2014, 57, 3546–3558. [Google Scholar] [CrossRef]
- Weiss, A.; van Beijnum, J.R.; Bonvin, D.; Jichlinski, P.; Dyson, P.J.; Griffioen, A.W.; Nowak-Sliwinska, P. Low-dose angiostatic tyrosine kinase inhibitors improve photodynamic therapy for cancer: Lack of vascular normalization. J. Cell. Mol. Med. 2014, 18, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Pion, E.; Asam, C.; Feder, A.-L.; Felthaus, O.; Heidekrueger, P.I.; Prantl, L.; Haerteis, S.; Aung, T. Laser speckle contrast analysis (LASCA) technology for the semiquantitative measurement of angiogenesis in in-ovo-tumor-model. Microvasc. Res. 2021, 133, 104072. [Google Scholar] [CrossRef] [PubMed]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 66018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briers, J.D.; Fercher, A.F. Retinal blood-flow visualization by means of laser speckle photography. Investig. Ophthalmol. Vis. Sci. 1982, 22, 255–259. [Google Scholar]
- Cyr, M.-P.; Pinard, A.; Dubois, O.; Morin, M. Reliability of vulvar blood perfusion in women with provoked vestibulodynia using laser Doppler perfusion imaging and laser speckle imaging. Microvasc. Res. 2019, 121, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.K.; Bolay, H.; Moskowitz, M.A.; Boas, D.A. Dynamic imaging of cerebral blood flow using laser speckle. J. Cereb. Blood Flow Metab. 2001, 21, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Ruaro, B.; Sulli, A.; Smith, V.; Paolino, S.; Pizzorni, C.; Cutolo, M. Short-term follow-up of digital ulcers by laser speckle contrast analysis in systemic sclerosis patients. Microvasc. Res. 2015, 101, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.; Schmidt, K.M.; Jordan, K.; Federlin, M.; Milenkovic, V.M.; Liebisch, G.; Artati, A.; Schmidl, C.; Madej, G.; Tokarz, J.; et al. Cancer-associated cells release citrate to support tumour metastatic progression. Life Sci. Alliance 2021, 4, e202000903. [Google Scholar] [CrossRef] [PubMed]
- Mycielska, M.E.; Milenkovic, V.M.; Wetzel, C.H.; Rümmele, P.; Geissler, E.K. Extracellular Citrate in Health and Disease. Curr. Mol. Med. 2015, 15, 884–891. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Dettmer, K.; Rümmele, P.; Schmidt, K.; Prehn, C.; Milenkovic, V.M.; Jagla, W.; Madej, G.M.; Lantow, M.; Schladt, M.; et al. Extracellular Citrate Affects Critical Elements of Cancer Cell Metabolism and Supports Cancer Development In Vivo. Cancer Res. 2018, 78, 2513–2523. [Google Scholar] [CrossRef] [Green Version]
- Mycielska, M.E.; Mohr, M.T.J.; Schmidt, K.; Drexler, K.; Rümmele, P.; Haferkamp, S.; Schlitt, H.J.; Gaumann, A.; Adamski, J.; Geissler, E.K. Potential Use of Gluconate in Cancer Therapy. Front. Oncol. 2019, 9, 522. [Google Scholar] [CrossRef]
- Kohl, C.; Aung, T.; Haerteis, S.; Papathemelis, T. Assessment of breast cancer primary tumor material in a 3D in vivo model. Clin. Hemorheol. Microcirc. 2021, 79, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, P.; Troeltzsch, M.; Brockmeyer, P.; Bohnenberger, H.; Heidekrüger, P.I.; Manzke, M.; Canis, M.; Gaayathiri, S.; Schliephake, H.; Prantl, L.; et al. First experience of chick chorioallantoic membrane (CAM) assay in the clinical work flow with oral squamous cell carcinoma patients. Clin. Hemorheol. Microcirc. 2018, 70, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Krump-Konvalinkova, V.; Bittinger, F.; Olert, J.; Bräuninger, W.; Brunner, J.; Kirkpatrick, C.J. Establishment and characterization of an angiosarcoma-derived cell line, AS-M. Endothelium 2003, 10, 319–328. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Medical Image Computing and Computer-Assisted Intervention-MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015: Proceedings, Part III; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Springer: Cham, Switzerland, 2015; pp. 234–241. ISBN 978-3-319-24573-7. [Google Scholar]
- Jiang, B.H.; Zheng, J.Z.; Aoki, M.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1749–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingenberg, M.; Becker, J.; Eberth, S.; Kube, D.; Wilting, J. The NADPH oxidase inhibitor imipramine-blue in the treatment of Burkitt lymphoma. Mol. Cancer Ther. 2014, 13, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtwright, A.; Siamakpour-Reihani, S.; Arbiser, J.L.; Banet, N.; Hilliard, E.; Fried, L.; Livasy, C.; Ketelsen, D.; Nepal, D.B.; Perou, C.M.; et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res. 2009, 69, 4621–4628. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.P.; Panigrahy, U.P.; Prasanth, D.; Gorla, U.S.; Guntupalli, C.; Panda, D.P.; Jena, B.R. A trimethoxy flavonoid isolated from stem extract of Tabebuia chrysantha suppresses angiogenesis in angiosarcoma. J. Pharm. Pharmacol. 2020, 72, 990–999. [Google Scholar] [CrossRef]
- Bader, A.G.; Kang, S.; Vogt, P.K. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 1475–1479. [Google Scholar] [CrossRef] [Green Version]
- Chin, W.W.L.; Thong, P.S.P.; Bhuvaneswari, R.; Soo, K.C.; Heng, P.W.S.; Olivo, M. In-vivo optical detection of cancer using chlorin e6--polyvinylpyrrolidone induced fluorescence imaging and spectroscopy. BMC Med. Imaging 2009, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Lecun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef] [Green Version]
- Albawi, S.; Mohammed, T.A.; Al-Zawi, S. Understanding of a convolutional neural network. In Proceedings of the 2017 International Conference on Engineering & Technology (ICET’2017), Antalya, Turkey, 21–23 August 2017; pp. 1–6. [Google Scholar]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Dong, J.; Li, H.; Gao, Y. Simple convolutional neural network on image classification. In Proceedings of the 2017 IEEE 2nd International Conference on Big Data Analysis (ICBDA 2017), Beijing, China, 10–12 March 2017; pp. 721–724. [Google Scholar]
- Lok, U.-W.; Huang, C.; Gong, P.; Tang, S.; Yang, L.; Zhang, W.; Kim, Y.; Korfiatis, P.; Blezek, D.J.; Lucien, F.; et al. Fast super-resolution ultrasound microvessel imaging using spatiotemporal data with deep fully convolutional neural network. Phys. Med. Biol. 2021, 66, 075005. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, L.; Zhang, J.; Huang, C.; Chen, S.; Luo, J. Localization of High-concentration Microbubbles for Ultrasound Localization Microscopy by Self-Supervised Deep Learning. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 November 2021; pp. 1–4. [Google Scholar]
- Chen, X.; Lowerison, M.R.; Dong, Z.; Han, A.; Song, P. Deep Learning-Based Microbubble Localization for Ultrasound Localization Microscopy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 1312–1325. [Google Scholar] [CrossRef]
- Humeau-Heurtier, A.; Guerreschi, E.; Abraham, P.; Mahé, G. Relevance of laser Doppler and laser speckle techniques for assessing vascular function: State of the art and future trends. IEEE Trans. Bio-Med. Eng. 2013, 60, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Zötterman, J.; Opsomer, D.; Farnebo, S.; Blondeel, P.; Monstrey, S.; Tesselaar, E. Intraoperative Laser Speckle Contrast Imaging in DIEP Breast Reconstruction: A Prospective Case Series Study. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2529. [Google Scholar] [CrossRef]
- Bamps, D.; Macours, L.; Buntinx, L.; de Hoon, J. Laser speckle contrast imaging, the future DBF imaging technique for TRP target engagement biomarker assays. Microvasc. Res. 2020, 129, 103965. [Google Scholar] [CrossRef]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. JBO 2010, 15, 11109. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuri, P.M.; Pion, E.; Mahl, L.; Kainz, P.; Schwarz, S.; Brochhausen, C.; Aung, T.; Haerteis, S. Deep Learning-Based Image Analysis for the Quantification of Tumor-Induced Angiogenesis in the 3D In Vivo Tumor Model—Establishment and Addition to Laser Speckle Contrast Imaging (LSCI). Cells 2022, 11, 2321. https://doi.org/10.3390/cells11152321
Kuri PM, Pion E, Mahl L, Kainz P, Schwarz S, Brochhausen C, Aung T, Haerteis S. Deep Learning-Based Image Analysis for the Quantification of Tumor-Induced Angiogenesis in the 3D In Vivo Tumor Model—Establishment and Addition to Laser Speckle Contrast Imaging (LSCI). Cells. 2022; 11(15):2321. https://doi.org/10.3390/cells11152321
Chicago/Turabian StyleKuri, Paulina Mena, Eric Pion, Lina Mahl, Philipp Kainz, Siegfried Schwarz, Christoph Brochhausen, Thiha Aung, and Silke Haerteis. 2022. "Deep Learning-Based Image Analysis for the Quantification of Tumor-Induced Angiogenesis in the 3D In Vivo Tumor Model—Establishment and Addition to Laser Speckle Contrast Imaging (LSCI)" Cells 11, no. 15: 2321. https://doi.org/10.3390/cells11152321