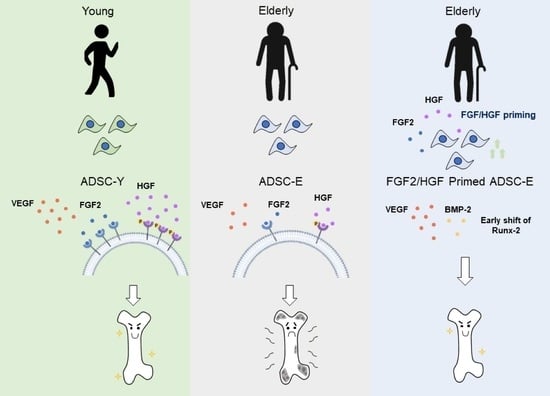
Priming with a Combination of FGF2 and HGF Restores the Impaired Osteogenic Differentiation of Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Osteogenic Induction and Growth Factor Treatment
2.3. Western Blot
2.4. Animals Model
2.5. Ectopic Bone Formation and Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. ADSCs with Weak Osteogenic Potential Is Deficient in Paracrine Factors under Normal Physiological Conditions
3.2. ADSCs with the Deficient Osteogenic Potential Shows Impaired Paracrine Action in Response to Osteogenic Stimulus
3.3. FGF2/HGF Priming Promotes Osteogenic Differentiation of ADSCs
3.4. FGF2/HGF Priming-Mediated Osteogenic Improvement Occurs by Modulation of Early Osteogenic Gene Expression
3.5. FGF2/HGF Priming Enhances the Bone-Forming Capacity of ADSCs In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lim, J.Y.; Ha, Y.C. Recent Epidemiology of Hip Fractures in South Korea. Hip Pelvis 2020, 32, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Tanha, K.; Fahimfar, N.; Nematollahi, S.; Sajjadi-Jazi, S.M.; Gharibzadeh, S.; Sanjari, M.; Khalagi, K.; Hajivalizedeh, F.; Raeisi, A.; Larijani, B.; et al. Annual incidence of osteoporotic hip fractures in Iran: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 668. [Google Scholar] [CrossRef] [PubMed]
- Ström, O.; Borgström, F.; Kanis, J.; Compston, J.; Cooper, C.; McCloskey, E.; Jönsson, B. Osteoporosis: Burden, health care provision and opportunities in the EU. Arch. Osteoporos. 2011, 6, 59–155. [Google Scholar] [CrossRef]
- Charkos, T.G.; Liu, Y.; Oumer, K.S.; Vuoung, A.M.; Yang, S. Effects of β-carotene intake on the risk of fracture: A Bayesian meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 711. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Poolman, R.W.; Agoritsas, T.; Siemieniuk, R.A.; Harris, I.A.; Schipper, I.B.; Mollon, B.; Smith, M.; Albin, A.; Nador, S.; Sasges, W.; et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: A clinical practice guideline. BMJ 2017, 356, j576. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell. Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.M.; Kim, B.-C.; Park, J.-H.; Kwon, I.K.; Mantalaris, A.; Hwang, Y.-S. Stem cells in bone tissue engineering. Biomed. Mater. 2010, 5, 062001. [Google Scholar] [CrossRef] [PubMed]
- Clines, G.A. Prospects for osteoprogenitor stem cells in fracture repair and osteoporosis. Curr. Opin. Organ. Transplant. 2010, 15, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.-R.; Jang, J.H.; Jeon, E.; Kang, W.; Lee, S.; Won, J.-E.; Kim, H.W.; Wall, I. Administration of growth factors for bone regeneration. Regen. Med. 2012, 7, 369–385. [Google Scholar] [CrossRef]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell. Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Emara, K.M.; Diab, R.A.; Emara, A.K. Recent biological trends in management of fracture non-union. World J. Orthop. 2015, 6, 623–628. [Google Scholar] [CrossRef]
- Gamblin, A.-L.; Brennan, M.A.; Renaud, A.; Yagita, H.; Lézot, F.; Heymann, D.; Trichet, V.; Layrolle, P. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: The local implication of osteoclasts and macrophages. Biomaterials 2014, 35, 9660–9667. [Google Scholar] [CrossRef] [Green Version]
- Watson, L.; Elliman, S.J.; Coleman, C.M. From isolation to implantation: A concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res. Ther. 2014, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.A.; Renaud, A.; Amiaud, J.; Rojewski, M.T.; Schrezenmeier, H.; Heymann, D.; Trichet, V.; Layrolle, P. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Res. Ther. 2014, 5, 114. [Google Scholar] [CrossRef] [Green Version]
- Chiba, Y.; Kuroda, S.; Osanai, T.; Shichinohe, H.; Houkin, K.; Iwasaki, Y. Impact of ageing on biological features of bone marrow stromal cells (BMSC) in cell transplantation therapy for CNS disorders: Functional enhancement by granulocyte-colony stimulating factor (G-CSF). Neuropathology 2012, 32, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Filion, T.M.; Skelly, J.D.; Huang, H.; Greiner, D.L.; Ayers, D.C.; Song, J. Impaired osteogenesis of T1DM bone marrow-derived stromal cells and periosteum-derived cells and their differential in-vitro responses to growth factor rescue. Stem Cell Res. Ther. 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.; Liu, W.; Peng, Z.; Lv, S.; Guan, Y.; An, G.; Zhang, Y.; Huang, T.; Wang, Y. The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qomi, R.T.; Sheykhhasan, M. Adipose-derived stromal cell in regenerative medicine: A review. World J. Stem Cells 2017, 9, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Heo, C.Y. Current applications of adipose-derived stem cells and their future perspectives. World J. Stem Cells 2014, 6, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Piao, J.; Park, G.; Yoo, K.S.; Hong, H.S. Osteoporotic Conditions Influence the Activity of Adipose-Derived Stem Cells. Tissue Eng. Regen. Med. 2020, 17, 875–885. [Google Scholar] [CrossRef]
- Zhang, Y.; Madhu, V.; Dighe, A.S.; Irvine, J.N.; Cui, Q. Osteogenic response of human adipose-derived stem cells to BMP-6, VEGF, and combined VEGF plus BMP-6 in vitro. Growth Factors 2012, 30, 333–343. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Guo, S.; He, Y.; Bai, G.; Zhang, W. Low-intensity pulsed ultrasound stimulation facilitates in vitro osteogenic differentiation of human adipose-derived stem cells via up-regulation of heat shock protein (HSP)70, HSP90, and bone morphogenetic protein (BMP) signaling pathway. Biosci. Rep. 2018, 38, BSR20180087. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zheng, Y.; Zhang, S.; Jia, L.; Zhou, Y. Promotion Effects of miR-375 on the Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rep. 2017, 8, 773–786. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, L.; Zhang, Y.; Gao, W.; Yao, Z.; Song, Y.; Wang, Y. Effects of age on biological and functional characterization of adipose-derived stem cells from patients with end-stage liver disease. Mol. Med. Rep. 2017, 16, 3510–3518. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.; Ryan, C.M.; Pathan, O.; Abraham, D.; Denton, C.P.; Butler, P.E. Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: Implications for regenerative medicine. Stem Cell Res. Ther. 2017, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Efimenko, A.; Dzhoyashvili, N.; Kalinina, N.; Kochegura, T.; Akchurin, R.; Tkachuk, V.; Parfyonova, Y. Adipose-Derived Mesenchymal Stromal Cells from Aged Patients With Coronary Artery Disease Keep Mesenchymal Stromal Cell Properties but Exhibit Characteristics of Aging and Have Impaired Angiogenic Potential. Stem Cell Transl. Med. 2014, 3, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, G.; Hong, H.S. Age affects the paracrine activity and differentiation potential of human adipose-derived stem cells. Mol. Med. Rep. 2021, 23, 160. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lei, H.; Dong, P.; Fu, X.; Yang, Z.; Yang, Y.; Ma, J.; Liu, X.; Cao, Y.; Xiao, R. Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell Transpl. 2017, 26, 1505–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Sterodimas, A.; de Faria, J.; Nicaretta, B.; Pitanguy, I. Tissue engineering with adipose-derived stem cells (ADSCs): Current and future applications. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1886–1892. [Google Scholar] [CrossRef]
- Lee, T.J.; Shim, M.S.; Yu, T.; Choi, K.; Kim, D.I.; Lee, S.H.; Bhang, S.H. Bioreducible polymer micelles based on acid-degradable Poly(ethylene glycol)-poly(amino ketal) enhance the stromal cell-derived factor-1α gene transfection efficacy and therapeutic angiogenesis of human adipose-derived stem cells. Int. J. Mol. Sci. 2018, 19, 529. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction−induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Gao, Y.; Dong, M.; Zheng, Z.; Li, Y.; Tan, R.; She, Z.; Yang, L. Epithelial differentiation of human adipose-derived stem cells (hASCs) undergoing three-dimensional (3D) cultivation with collagen sponge scaffold (CSS) via an indirect co-culture strategy. Stem Cell Res. Ther. 2020, 11, 141. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Mazini, L.; Ezzoubi, M.; Malka, G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: Flow chart and regulation updates before and after COVID-19. Stem Cell Res. Ther. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Shi, G.; Lei, X.; Huang, Y.; Li, X.; Bai, L.; Qin, C. Age-related alteration in characteristics, function, and transcription features of ADSCs. Stem Cell Res. Ther. 2021, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, H.; Kalarestaghi, H. Adipose-derived stem cells: An appropriate selection for osteogenic differentiation. J. Cell Physiol. 2020, 235, 8371–8386. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Zhang, C.; Chen, G.; Tang, Z.; Liu, Q.; Chen, J.; Tong, X.; Wang, J. Effects of BMP-2 and FGF2 on the Osteogenesis of Bone Marrow-Derived Mesenchymal Stem Cells in Hindlimb-Unloaded Rats. Cell Biochem. Biophys. 2014, 70, 1127–1136. [Google Scholar] [CrossRef]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef]
- Sun, Z.; Tee, B.C.; Kennedy, K.S.; Kennedy, P.M.; Kim, D.-G.; Mallery, S.R.; Fields, H.W. Scaffold-based delivery of autologous mesenchymal stem cells for mandibular distraction osteogenesis: Preliminary studies in a porcine model. PLoS ONE 2013, 8, e74672. [Google Scholar] [CrossRef] [Green Version]
- Hurley, M.M.; Gronowicz, G.; Zhu, L.; Kuhn, L.T.; Rodner, C.; Xiao, L. Age-related changes in FGF-2, fibroblast growth factor receptors and β-Catenin expression in human mesenchyme-derived progenitor cells. J. Cell Biochem. 2016, 117, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.S.; Volk, C.; Marga, C.; Navarrete Santos, A.; Jung, M.; Rujescu, D.; Navarrete, S.A. Adipose-derived stem/stromal cells recapitulate aging biomarkers and show reduced stem cell plasticity affecting their adipogenic differentiation capacity. Cell Reprogram. 2019, 21, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Kim, S.; Cui, X.; Zheng, L.; Oh, S.J.; Anderson, T.; AlSharabati, M.; Kazamel, M.; Volpicelli-Daley, L.; Bamman, M.M.; et al. Transforming growth gactor beta (TGF-β) is a muscle biomarker of disease progression in ALS and correlates with Smad Expression. PLoS ONE 2015, 10, e0138425. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, V.; Pourheydar, B.; Dariushnejad, H.; Ghalibafsabbaghi, A.; Chodari, L. Curcumin improves angiogenesis in the heart of aged rats: Involvement of TSP1/NF-κB/VEGF-A signaling. Microvasc. Res. 2022, 139, 104258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhen, R.; Yang, J.; Wang, Y.; Li, Y.; Chen, B.; Song, Y.; Ma, G.; Yang, B. Hepatocyte growth factor improves bone regeneration via the bone morphogenetic protein-2-mediated NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 6045–6053. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.Y.; Huang, Y.-L.; Yang, W.-H.; Tang, C.-H. Hepatocyte growth factor-induced BMP-2 expression is mediated by c-Met receptor, FAK, JNK, Runx2, and p300 pathways in human osteoblasts. Int. Immunopharmacol. 2012, 13, 156–162. [Google Scholar] [CrossRef]
- Lin, Y.M.; Huang, Y.L.; Fong, Y.C.; Tsai, C.-H.; Chou, M.C.; Tang, C.H. Hepatocyte growth factor increases vascular endothelial growth factor-A production in human synovial fibroblasts through c-Met receptor pathway. PLoS ONE 2012, 7, e50924. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Okamura, H.; Qiu, L. Upregulated osterix expression elicited by Runx2 and Dlx5 is required for the accelerated osteoblast differentiation in PP2A Cα-knockdown cells. Cell Biol. Int. 2018, 42, 403–410. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of Aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Kim, D.; Hong, H.S. Priming with a Combination of FGF2 and HGF Restores the Impaired Osteogenic Differentiation of Adipose-Derived Stem Cells. Cells 2022, 11, 2042. https://doi.org/10.3390/cells11132042
Park JS, Kim D, Hong HS. Priming with a Combination of FGF2 and HGF Restores the Impaired Osteogenic Differentiation of Adipose-Derived Stem Cells. Cells. 2022; 11(13):2042. https://doi.org/10.3390/cells11132042
Chicago/Turabian StylePark, Jeong Seop, Doyoung Kim, and Hyun Sook Hong. 2022. "Priming with a Combination of FGF2 and HGF Restores the Impaired Osteogenic Differentiation of Adipose-Derived Stem Cells" Cells 11, no. 13: 2042. https://doi.org/10.3390/cells11132042