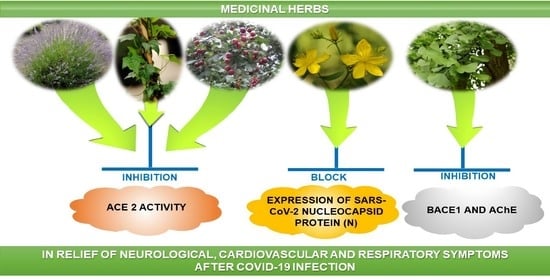
Medicinal Herbs in the Relief of Neurological, Cardiovascular, and Respiratory Symptoms after COVID-19 Infection A Literature Review
Abstract
:1. Introduction
COVID-19 Related Complications in Humans
2. Phytotherapy of the Central Nervous System
2.1. Plant Raw Materials with an Anxiolytic Effect
2.2. Phytotherapy of Depression
2.3. Herbal Medicines in the Mixed Anxiety–Depressive Syndrome
2.4. Medicinal Plants That Improve Memory Processes and Cognitive Functions
3. Phototherapy of Cardiovascular and Heart Disorders as a Result of Neurological Symptoms in COVID-19 Infection
4. Herbal Raw Materials That Inhibit Inflammation of the Respiratory Tract Caused by COVID-19 Infection
| Medicinal Herbs | Active Molecule | Structure | Mechanism |
|---|---|---|---|
| Valerianae radix | Valeranone |  | Extract significantly decreased 3H-glutamate binding [108] |
| Valtrate |  | Extract influences NMDA and AMPA receptor binding [108] | |
| Melissae folium | Apigenin |  | Interaction with both S 1 and S2 domains of the spike protein of SARS-CoV-2Binding affinity with the SARS-CoV-2 major protease (6 LU7) [109] |
| Melissae flos | Citronellal |  | Inhibition of ACE2 activity [110] |
| Passiflorae herba | Isovitexin |  | SARS-CoV-2 main protease inhibitor [111] |
| Isoorientin |  | ||
| Piperis methystici rhizoma (kava-kava) | Methysticin |  | SARS-CoV-2 main protease inhibitor [112] |
| Lupuli flos | Humulone |  | Act as immunomodulator [113] |
| Farnesene |  | ||
| Ballotae nigrae herba | Acteoside |  | SARS-CoV-2 main protease inhibitor [114] |
| Hyperici herba | Hypericin |  | Block of the expression of the SARS-CoV-2 nucleocapsid protein (N) [115] |
| Hyperforin |  | ||
| Biapigenin |  | Block of the expression of the SARS-CoV-2 nucleocapsid protein (N) [115] | |
| Rhodiolae radix | Rosavidin |  | Transcriptional control of metabolic regulation [116] |
| Salidroside |  | ||
| Lavandulae flos | Linalool acetate |  | Inhibition of ACE2 activity [110] |
| Linalool |  | ||
| Pauliniae semen(guarana) | Caffeine |  | Inhibition of ACE2 activity [117] |
| Ginkgo folium | Ginkgolide |  | |
| Bilobalide |  | Inhibition of the targeting protein and DNA [118] | |
| Murrayae folium | Mahanimbine |  | Inhibition BACE1 and AChE, Decrease IL-1 β and TNF-α, COX2, Increase TGF-β and IL-10 [119] |
| Crataegi fructus | Epicatechin |  | Inhibition of ACE2 activity [120] |
| Hederae helicis folium | Hederacoside C |  | Inhibition of ACE2 activity [121] |
| α- hederine |  | ||
| Polygalae radix | Presegenin |  | An inhibitor of both S and ACE2 proteins [122] |
| Pelargonii radix | Umckalin |  | |
| 6,8-Bis(sulfooxy)-7-methoxy-2H-1-benzopyran-2-one |  | Affects propagation [123] | |
| Lichen islandicus | Fumaroprotocetric acid |  | Stimulate an increase of NO release in macrophages [124] |
| Protolichesteric acid |  | ||
| Plantaginis lanceolatae folium et herba | Aucubin |  | Inhibition of TNF-alpha and IL-6 production [125] |
| Acteoside |  |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choi, J.; Min, S.Y.; Kim, J.H.; Jeong, A. Efficacy of traditional herbal medicine for psychological sequelae in COVID-19 survivors: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25609. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Steele, B.; Bryant, J.; Fouad, E.; Toyang, N.; Ngwa, W. Antiviral Activity of Jamaican Medicinal Plants and Isolated Bioactive Compounds. Molecules 2021, 26, 607. [Google Scholar] [CrossRef]
- Am, S.; Em, A. Medicinal Plants with Antiviral Properties to Tackle COVID-19 Pandemic: A Short-Review. SunText Rev. Biotechnol. 2021, 2, 122. [Google Scholar] [CrossRef]
- Salem, M.A.; Ezzat, S.M. The use of aromatic plants and their therapeutic potential as antiviral agents: A hope for finding anti-COVID 19 essential oils. J. Essent. Oil Res. 2021, 33, 105–113. [Google Scholar] [CrossRef]
- Khan, T.; Khan, M.A.; Mashwani, Z.-U.; Ullah, N.; Nadhman, A. Therapeutic potential of medicinal plants against COVID-19: The role of antiviral medicinal metabolites. Biocatal. Agric. Biotechnol. 2020, 31, 101890. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight Into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef]
- Demeke, C.A.; Woldeyohanins, A.E.; Kifle, Z.D. Herbal medicine use for the management of COVID-19: A review article. Metab. Open 2021, 12, 100141. [Google Scholar] [CrossRef]
- Tsai, K.-C.; Huang, Y.-C.; Liaw, C.-C.; Tsai, C.-I.; Chiou, C.-T.; Lin, C.-J.; Wei, W.-C.; Lin, S.J.-S.; Tseng, Y.-H.; Yeh, K.-M.; et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: A bedside-to-bench study. Biomed. Pharmacother. 2020, 133, 111037. [Google Scholar] [CrossRef]
- Ning, Q.; Wu, D.; Wang, X.; Xi, D.; Chen, T.; Chen, G.; Wang, H.; Lu, H.; Wang, M.; Zhu, L.; et al. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduct. Target. Ther. 2022, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, T.; Feng, J. Complications and Pathophysiology of COVID-19 in the Nervous System. Front. Neurol. 2020, 11, 573421. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Du, T.; Hong, W.; Chen, L.; Que, H.; Lu, S.; Peng, X. Neurological complications and infection mechanism of SARS-CoV-2. Signal Transduct. Target. Ther. 2021, 6, 406. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Sanders, E.C. Potential neuroinvasive pathways of SARS-CoV-2: Deciphering the spectrum of neurological deficit seen in coronavirus disease 2019 (COVID-19). J. Med. Virol. 2020, 92, 1845–1857. [Google Scholar] [CrossRef]
- DosSantos, M.F.; Devalle, S.; Aran, V.; Capra, D.; Roque, N.R.; Coelho-Aguiar, J.D.M.; Spohr, T.C.L.D.S.E.; Subilhaga, J.G.; Pereira, C.M.; Meira, I.D.; et al. Neuromechanisms of SARS-CoV-2: A Review. Front. Neuroanat. 2020, 14, 37. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef]
- Matías-Guiu, J.; Gomez-Pinedo, U.; Montero-Escribano, P.; Gomez-Iglesias, P.; Porta-Etessam, J.; Matias-Guiu, J.A. Should we expect neurological symptoms in the SARS-CoV2 epidemic? Neurologia 2020, 35, 170–175. [Google Scholar] [CrossRef]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021, 11, 573095. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother. Res. 2021, 35, 1237–1247. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2020, 1486, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Boulenger, J.P.; Lavallée, Y.J. Mixed anxiety and depression: Diagnostic issues. J. Clin. Psychiatry 1993, 54, 3–8. [Google Scholar] [PubMed]
- Thun, E.; Sivertsen, B.; Knapstad, M.; Smith, O.R. Unravelling the Prospective Associations Between Mixed Anxiety-Depression and Insomnia During the Course of Cognitive Behavioral Therapy. Psychosom. Med. 2019, 81, 333–340. [Google Scholar] [CrossRef]
- Philbert, M.A.; Billingsley, M.L.; Reuhl, K.R. Mechanisms of Injury in the Central Nervous System. Toxicol. Pathol. 2000, 28, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Berlińska, A.; Świątkowska-Stodulska, R.; Sworczak, K. Old Problem, New Concerns: Hypercortisolemia in the Time of COVID-19. Front. Endocrinol. 2021, 12, 711612. [Google Scholar] [CrossRef]
- Gajcy, K.; Lochynski, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347. [Google Scholar] [CrossRef]
- Tian, J.; Middleton, B.; Kaufman, D. GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses 2021, 13, 966. [Google Scholar] [CrossRef]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef]
- Mineo, L.; Concerto, C.; Patel, D.; Mayorga, T.; Paula, M.; Chusid, E.; Aguglia, E.; Battaglia, F. Valeriana officinalis Root Extract Modulates Cortical Excitatory Circuits in Humans. Neuropsychobiology 2017, 75, 46–51. [Google Scholar] [CrossRef]
- Hendriks, H.; Bos, R.; Allersma, D.P.; Malingré, T.M.; Koster, A.S. Pharmacological Screening of Valerenal and some other Components of Essential Oil of Valeriana officinalis. Planta Med. 1981, 42, 62–68. [Google Scholar] [CrossRef]
- Valerianae Radix. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 539–546.
- Hattesohl, M.; Feistel, B.; Sievers, H.; Lehnfeld, R.; Hegger, M.; Winterhoff, H. Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine 2008, 15, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckann, J. Valerian root. In Herbal Medicine. Expanded Commission E Monographs, 1st ed.; American Botanical Council: Austin, TX, USA, 2000; pp. 394–400. [Google Scholar]
- European Medicines Agency. Human Regulatory. In Science Medicines Health; Valeriana officinalis L. Radix; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Koch-Heitzmann, R.; Schultze, W. 2000 Jahre Melissa officinalis. Zeitschiftfur Phytother. 1988, 9, 77–85. [Google Scholar]
- Blumenthal, M.; Goldberg, A.; Brinckann, J. Lemon balm. In Herbal Medicine. Expanded Commission E Monographs, 1st ed.; American Botanical Council: Austin, TX, USA, 2000; pp. 250–252. [Google Scholar]
- Melissae folium. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 324–327.
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Melissa officinalis L. Folium; European Medicines Agency: London, UK, 2013. [Google Scholar]
- Akhondzadeh, S.; Naghavi, H.R.; Vazirian, M.; Shayeganpour, A.; Rashidi, H.; Khani, M. Passionflower in the treatment of generalized anxiety: A pilot double-blind randomized controlled trial with oxazepam. J. Clin. Pharm. Ther. 2001, 26, 363–367. [Google Scholar] [CrossRef]
- Galiano, G.; Foussard-Blampin, O.; Bretaudeau, J. Etude experimentale du role dans les properietes psycho-pharmacologiques de Passiflora incarnate L. Phytotherapy 1994, 40–41, 18–22. [Google Scholar]
- Speroni, E.; Billi, R.; Mercati, V.; Boncompagni, E.; Toja, E. Sedative effects of crude extract of Passiflora incarnata after oral administration. Phytother. Res. 1996, 10, 592–594. [Google Scholar]
- Passiflorae Herba. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 359–364.
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Passiflora incarnata L. Herba; European Medicines Agency: London, UK, 2007. [Google Scholar]
- Gessner, B.; Cnota, P. Extract of kava-kava rhizome in comparison with diazepam and placebo. Z. Phtytother. 1994, 15, 30–37. [Google Scholar]
- Gleitz, J.; Beile, A.; Peters, T. (+,−)-Kavain inhibits veratridine activated and KCl induced increase in intracellular Ca2+ and glutamate-release of rat cerebrocorticalsynaptosomes. Neuropharmacology 1996, 315, 179–186. [Google Scholar] [CrossRef]
- Heinze, H.J.; Münthe, T.F.; Steitz, J.; Matzke, M. Pharmacopsychological Effects of Oxazepam and Kava-Extract in a Visual Search Paradigm assessed with Event-Related Potentials. Pharmacopsychiatry 1994, 27, 224–230. [Google Scholar] [CrossRef]
- Lehmann, E.; Kinzler, E.; Friedmann, J. Efficacy of a special kava extract (Piper mathysticum), in patient with states of anxiety, tension and excitedness, of a nonmental origin: A double-blind placebo controlled study of four weeks treatment. Phytomedicine 1996, 3, 113–119. [Google Scholar] [CrossRef]
- Volz, H.P.; Kiesser, M. Kava-kava extract WS 1490 versus placebo in anxiety disorders: A randomized placebo-controlled 25 weeks outpatient trial. Pharmacopsychiatry 1997, 30, 1–5. [Google Scholar] [CrossRef]
- Piperis Methystici rhizome. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 365–382.
- Wohlfart, R.; Hansel, R.; Schmidt, H. Nachweis sedative-hypnotischerWirkstoffeimHopfen. 4. Mitteilung. Die Pharmakologie des Hopfeninhalssstofles 2-Methyl-3-buten-2-ol. Planta Med. 1983, 48, 120–123. [Google Scholar] [CrossRef]
- Lupuli flos. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 306–311.
- Kumar, S.; Kumari, R. Pharmacological Activities of Ballota nigra (L.) Benth: A Mini Review. Int. J. Pharma Med. Biol. Sci. 2021, 10, 114–119. [Google Scholar] [CrossRef]
- European Scientific Cooperative on Phytotherapy (ESCOP). Ballotae Nigrae Herba—Black Horehound—MONOGRAPHS. Available online: https://escop.com/wp-content/uploads/edd/2016/06/Ballota.pdf (accessed on 25 April 2022).
- Wonnemann, M.; Singer, A.; Müller, W.E. Inhibition of Synaptosomal Uptake of 3H-L-glutamate and 3H-GABA by Hyperforin, a Major Constituent of St. John’s Wort The Role of Amiloride Sensitive Sodium Conductive Pathways. Neuropsychopharmacology 2000, 23, 188–197. [Google Scholar] [CrossRef]
- Kaehler, S.T.; Sinner, C.; Chatterjee, S.S.; Philippu, A. Hyperforin enhances the extracellular concentrations of catecholamines, serotonin and glutamate in the rat locus coeruleus. Neurosci. Lett. 1999, 262, 199–202. [Google Scholar] [CrossRef]
- Hyperici Herba. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 257–281.
- Thiede, H.-M.; Walper, A. MAO- und COMT- Hemmungdurch Hypericum-Extracte. Nevenheilkunde 1993, 12, 346–348. [Google Scholar]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. A review on antidepressant plants. Nat. Prod. Radiance 2006, 5, 144–152. [Google Scholar]
- Ishaque, S.; Shamseer, L.; Bukutu, C.; Vohra, S. Rhodiola rosea for physical and mental fatigue: A systematic review. BMC Complement. Altern. Med. 2012, 12, 70. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Orhan, I.E.; Badiee, A.; Daglia, M. Rhodiola rosea L. and Alzheimer’s Disease: From Farm to Pharmacy. Phytother. Res. 2016, 30, 532–539. [Google Scholar] [CrossRef]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Rhodiola Rosea L. Rhizoma et Radix; European Medicines Agency: London, UK, 2012. [Google Scholar]
- Stein, M.B.; Kirk, P.; Prabhn, V. Mixed anxiety-depression in a primary care clinic. J. Affect Disord. 1995, 34, 78–94. [Google Scholar] [CrossRef]
- World Health Organization. ICD-10, International Statistical Classification of Disearse and Related Health Problems; Tenth Revision; WHO: Geneva, Switzerland, 1992.
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of Lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The Effects of Lavender Essential Oil on Wound Healing: A Review of the Current Evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Sasannejad, P.; Saeedi, M.; Shoeibi, A.; Gorji, A.; Abbasi, M.; Foroughipour, M. Lavender Essential Oil in the Treatment of Migraine Headache: A Placebo-Controlled Clinical Trial. Eur. Neurol. 2012, 67, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Lavandulae flos/aetheroleum. In ESCOP Monographs, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2009; pp. 147–156.
- Moustakas, D.; Mezzio, M.; Rodriguez, B.R.; Constable, M.A.; Mulligan, M.E.; Voura, E.B. Guarana Provides Additional Stimulation over Caffeine Alone in the Planarian Model. PLoS ONE 2015, 10, e0123310. [Google Scholar] [CrossRef]
- Pomportes, L.; Brisswalter, J.; Hays, A.; Davranche, K. Effects of Carbohydrate, Caffeine, and Guarana on Cognitive Performance, Perceived Exertion, and Shooting Performance in High-Level Athletes. Int. J. Sports Physiol. Perform. 2019, 14, 576–582. [Google Scholar] [CrossRef]
- Pauliniae semen/Guarana seeds. In ESCOP Monographs, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2009; pp. 247–256.
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Paulinia cupana Kunth ex K.B.H. var. Sorbilis. Ducke, Semen; European Medicines Agency: London, UK, 2012. [Google Scholar]
- Alonso-Lana, S.; Marquié, M.; Ruiz, A.; Boada, M. Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals With Dementia. Front. Aging Neurosci. 2020, 12, 588872. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Res. Ther. 2020, 12, 69. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ramassamy, C.; Doré, S.; Christen, Y.; Poirier, J.; Quirion, R. The ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur. J. Neurosci. 2000, 12, 1882–1890. [Google Scholar] [CrossRef]
- Stough, C.; Clarke, J.; Lloyd, J.; Nathan, P.J. Neuropsychological changes after 30-day Ginkgo biloba administration in healthy participants. Int. J. Neuropsychopharmacol. 2001, 4, 131–134. [Google Scholar] [CrossRef]
- Ernst, E.; Pittler, M.H. Ginkgo biloba for dementia. A systematic review of double-blind, placebo-controlled trials. Clin. Drug Investig. 1999, 17, 301–308. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ginkgo folium. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 178–210.
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Ginkgo biloba L. Folium; European Medicines Agency: London, UK, 2015. [Google Scholar]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Liu, X. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016, 193, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Eslinger, P.J.; Doty, R.L.; Zimmerman, E.K.; Grunfeld, R.; Sun, X.; Meadowcroft, M.D.; Connor, J.R.; Price, J.L.; Smith, M.B.; et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010, 1357, 184–194. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Lavandula Angustifolia Miller, Flos; European Medicines Agency: London, UK, 2012. [Google Scholar]
- Kennedy, D.; Little, W.; Scholey, A. Attenuation of Laboratory-Induced Stress in Humans After Acute Administration of Melissa officinalis (Lemon Balm). Psychosom. Med. 2004, 66, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; O’Brien, J.T.; Reichelt, K.; Perry, E.K. Aromatherapy as a Safe and Effective Treatment for the Management of Agitation in Severe Dementia. J. Clin. Psychiatry 2002, 63, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Scholey, A.; Tildesley, N.; Perry, E.; Wesnes, K. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol. Biochem. Behav. 2002, 72, 953–964. [Google Scholar] [CrossRef]
- Mahipal, P.; Pawar, R.S. Nephroprotective effect of Murraya koenigii on cyclophosphamide induced nephrotoxicity in rats. Asian Pac. J. Trop. Med. 2017, 10, 808–812. [Google Scholar] [CrossRef]
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 247–250. [Google Scholar] [CrossRef]
- Orth-Wagner, S.; Scheurich, E. Moderne Phytotherapie. Teil IV: Weissdorn (Crataegus). Der. Deutsche Apotheker Heft. 1986, 11, 387–390. [Google Scholar]
- Schwinger, R.H.G.; Pietsch, M.; Frank, K.; Brixius, K. Crataegus Special Extract WS 1442 Increases Force of Contraction in Human Myocardium cAMP-Independently. J. Cardiovasc. Pharmacol. 2000, 35, 700–707. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Koch, E.; Jaggy, H.; Krzeminski, T. In-vitro- Und In-vivo-UntersuchungenzurkardioprotektivenWirkung von oligomerenProcyanidinenIneinemCrataegus-ExtraktausBlätternmitBlüten. Arzneimittel-Forschung 1997, 47, 821–825. [Google Scholar]
- Schüssler, M.; Hölzl, J.; Fricke, U. Myocardial effects of flavonoids from Crataegus species. Arzneimittel-Forschung 1995, 45, 842–845. [Google Scholar]
- Schmidt, U.; Albrecht, M.; Podzuweit, H.; Ploch, M.; Maisenbacher, J. Hochdosierte Crataegus-Therapie bei herzinsuffizienten Patienten NYHA-Stadium I und II. Z. Phytother. 1998, 19, 22–30. [Google Scholar]
- ESCOP Monographs, 2nd ed.; Crataegi fructus; Thieme Publisher: Stuttgart, NY, USA, 2009; pp. 45–49.
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2020, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Sieben, A.; Prenner, L.; Sorkalla, T.; Wolf, A.; Jakobs, D.; Runkel, F.; Häberlein, H. α-Hederin, but Not Hederacoside C and Hederagenin from Hedera helix, Affects the Binding Behavior, Dynamics, and Regulation of β2-Adrenergic Receptors. Biochemistry 2009, 48, 3477–3482. [Google Scholar] [CrossRef]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Hedera helix, L. Folium; European Medicines Agency: London, UK, 2010. [Google Scholar]
- Zhao, X.; Cui, Y.; Wu, P.; Zhao, P.; Zhou, Q.; Zhang, Z.; Wang, Y.; Zhang, X. Polygalae Radix: A review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 2020, 147, 104759. [Google Scholar] [CrossRef] [PubMed]
- Polygalae radix. In ESCOP Monographs: European Scientific Cooperative on Phytotherapy, 2nd ed.; Thieme Publisher: Stuttgart, NY, USA, 2003; pp. 400–406.
- Conrad, A.; Frank, U. Extract of Pelargonium sidoides (EPs® 7630) Displays Anti-Infective Properties by Enhanced Phagocytosis and Differential Modulation of Host-Bacteria Interactions. Plant. Med. 2008, 74, 682–685. [Google Scholar] [CrossRef]
- Bachert, C.; Schapowal, A.; Funk, P.; Kieser, M. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: A randomized, double-blind, placebo-controlled trial. J. App. Microbiol. 2009, 106, 1184–1193. [Google Scholar]
- Golovatiuk, A.; Tschtschalin, A.G. WirsamkeiteinenEkstraktenausPelargonium sidoides (EPS 7630) versus Placebo beiaktuer Bronchitis. In Phytopharmaka VII—Forschung und Klinischeanwendung; Schulz, V., Reitbrok, N., Roots, I., Loew, D., Eds.; Steinkopff: Darmstadt, Germany, 2002; pp. 3–12. [Google Scholar]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Pelargonium sidoides Kurt. Radix; European Medicines Agency: London, UK, 2015. [Google Scholar]
- Ingolfsdotir, K.; Breu, W.; Huneck, S.; Gudjonsdotir, C.A.; Miller-Jakic, B.; Wagner, H. In vitro inhibition of 5-lipooxygenase by protolichesterenic acid fromCetrariaIslandica (Iceland moss). Phytomedicine 1998, 5, 333–339. [Google Scholar]
- Kumar, K.C.S.; Müller, K. Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J. Nat. Prod. 1999, 62, 817–820. [Google Scholar] [CrossRef]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Cetrariaislandica L./Achariuss L., Thallus; European Medicines Agency: London, UK, 2014. [Google Scholar]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Plantago lanceolata L., Folium; European Medicines Agency: London, UK, 2014. [Google Scholar]
- Lundstrom, K.; Pham, H.T.; Dinh, L.D. Interaction of Plant Extracts with Central Nervous System Receptors. Medicines 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Oladele, J.O.; Oladele, O.T.; Oyeleke, O.M.; Oladiji, A.T. Neurological Complications in COVID-19: Implications on International Health Security and Possible Interventions of Phytochemicals. In Contemporary Developments and Perspectives in International Health Security; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wang, C.-S.; Chen, C.-C.; Chen, Y.-C.; Lu, L.-P.; Huang, C.-H.; Lai, C.-S.; Wang, S.-Y. Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, S.; Yalçınkaya, S.; Ercan, F. In silico detection of inhibitor potential of Passiflora compounds against SARS-Cov-2(COVID-19) main protease by using molecular docking and dynamic analyses. J. Mol. Struct. 2021, 1240, 130556. [Google Scholar] [CrossRef]
- Davella, R.; Gurrapu, S.; Mamidala, E. Phenolic compounds as promising drug candidates against COVID-19—An integrated molecular docking and dynamics simulation study. Mater. Today Proc. 2021, 51, 522–527. [Google Scholar] [CrossRef]
- Lucas, K.; Fröhlich-Nowoisky, J.; Oppitz, N.; Ackermann, M. Cinnamon and Hop Extracts as Potential Immunomodulators for Severe COVID-19 Cases. Front. Plant Sci. 2021, 12, 589783. [Google Scholar] [CrossRef] [PubMed]
- Kallingal, A.; Kundil, V.T.; Ayyolath, A.; Karlapudi, A.P.; Joseph, T.M.; Jayadevi, V. Molecular modeling study of tectoquinone and acteoside from Tectona grandis linn: A new SARS-CoV-2 main protease inhibitor against COVID-19. J. Biomol. Struct. Dyn. 2020, 40, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.F.; Anhlan, D.; Schöfbänker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kühn, J.; Hrincius, E.R.; et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef]
- Karosanidze, I.; Kiladze, U.; Kirtadze, N.; Giorgadze, M.; Amashukeli, N.; Parulava, N.; Iluridze, N.; Kikabidze, N.; Gudavadze, N.; Gelashvili, L.; et al. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15, 345. [Google Scholar] [CrossRef]
- Romero-Martínez, B.S.; Montaño, L.M.; Solís-Chagoyán, H.; Sommer, B.; Ramírez-Salinas, G.L.; Pérez-Figueroa, G.E.; Flores-Soto, E. Possible Beneficial Actions of Caffeine in SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 5460. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Ramadan, H.H.; Mohammed, R.N. Evidence that Ginkgo Biloba could use in the influenza and coronavirus COVID-19 infections. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 131–143. [Google Scholar] [CrossRef]
- Tan, M.A.; Sharma, N.; An, S.S.A. Multi-Target Approach of Murraya koenigii Leaves in Treating Neurodegenerative Diseases. Pharmaceuticals 2022, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Al-Shuhaib, M.B.S.; Hashim, H.O.; Al-Shuhaib, J.M. Epicatechin is a promising novel inhibitor of SARS-CoV-2 entry by disrupting interactions between angiotensin-converting enzyme type 2 and the viral receptor binding domain: A computational/simulation study. Comput. Biol. Med. 2021, 141, 105155. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Bi, S.W.; Gu, J.; Wu, T.Y.; He, L.L. Screening novel inhibitors targeting SARS-CoV-2 S protein-ACE2 interaction based on molecular docking. Chin. Tradit. Herb. Drugs 2020, 51, 2361–2367. [Google Scholar]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 581840. [Google Scholar] [CrossRef] [PubMed]
- Papies, J.; Emanuel, J.; Heinemann, N.; Kulić, Ž.; Schroeder, S.; Tenner, B.; Lehner, M.D.; Seifert, G.; Müller, M.A. Corrigendum: Antiviral and Immunomodulatory Effects of Pelargonium sidoides DC. Root Extract EPs® 7630 in SARS-CoV-2-Infected Human Lung Cells. Front. Pharmacol. 2021, 12, 814452. [Google Scholar] [CrossRef]
- Santos, L.; Honda, N.; Carlos, I.Z.; Vilegas, W. Intermediate reactive oxygen and nitrogen from macrophages induced by Brazilian lichens. Fitoterapia 2004, 75, 473–479. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Koo, H.-N.; Na, H.-J.; Kim, M.-S.; Hong, S.-H.; Eom, J.-W.; Kim, K.-S.; Shin, T.-Y.; Kim, H.-M. Inhibition of Tnf-A And Il-6 Production by Aucubin through Blockade of Nf-Κb Activation in Rbl-2h3 Mast Cells. Cytokine 2002, 18, 252–259. [Google Scholar] [CrossRef]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Lavandula angustifolia Miller, aetheroleum; European Medicines Agency: London, UK, 2012. [Google Scholar]
- European Medicines Agency. Human Regulatory. In Community Herbal Monograph on Crataegus spp.; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Blumenthal, M.; Goldberg, A.; Brinckann, J. Ivy Leaf. In Herbal Medicine. Expanded Commission E Monographs, 1st ed.; American Botanical Council: Austin, TX, USA, 2000; pp. 215–217. [Google Scholar]
- Wyganowska-Swiatkowska, M.; Nohawica, M.; Grocholewicz, K.; Nowak, G. Influence of Herbal Medicines on HMGB1 Release, SARS-CoV-2 Viral Attachment, Acute Respiratory Failure, and Sepsis. A Literature Review. Int. J. Mol. Sci. 2020, 21, 4639. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Nawrot, J.; Budzianowski, J.; Nowak, G.; Micek, I.; Budzianowska, A.; Gornowicz-Porowska, J. Biologically Active Compounds in Stizolophus balsamita Inflorescences: Isolation, Phytochemical Characterization and Effects on the Skin Biophysical Parameters. Int. J. Mol. Sci. 2021, 22, 4428. [Google Scholar] [CrossRef]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Llivisaca-Contreras, S.; Naranjo-Morán, J.; Pino-Acosta, A.; Pieters, L.; Berghe, W.V.; Manzano, P.; Vargas-Pérez, J.; León-Tamariz, F.; Cevallos-Cevallos, J. Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2. Molecules 2021, 26, 4099. [Google Scholar] [CrossRef] [PubMed]
- Jelaska, S.; Mihaljević, S.; Bauer, N. Production of biopharmaceuticals, antibodies and edible vaccines in transgenic plants. Curr. Stud. Biotechnol. 2005, 4, 121–127. [Google Scholar]
- Silwa, S.M.S.P.; Siridewa, K. Molecular Pharming: A New Approach for a Healthy Future by a Vast Development in the Pharmaceutical Industry. Curr. Trends Biotechnol. Pharm. 2021, 15, 315–324. [Google Scholar] [CrossRef]
- Ayan, A.; Meriç, S.; Gümüş, T.; Atak, Ç. Next Generation of Transgenic Plants: From Farming to Pharming. In Genetically Modified Plants and Beyond; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/chapters/80206 (accessed on 29 March 2022). [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrot, J.; Gornowicz-Porowska, J.; Budzianowski, J.; Nowak, G.; Schroeder, G.; Kurczewska, J. Medicinal Herbs in the Relief of Neurological, Cardiovascular, and Respiratory Symptoms after COVID-19 Infection A Literature Review. Cells 2022, 11, 1897. https://doi.org/10.3390/cells11121897
Nawrot J, Gornowicz-Porowska J, Budzianowski J, Nowak G, Schroeder G, Kurczewska J. Medicinal Herbs in the Relief of Neurological, Cardiovascular, and Respiratory Symptoms after COVID-19 Infection A Literature Review. Cells. 2022; 11(12):1897. https://doi.org/10.3390/cells11121897
Chicago/Turabian StyleNawrot, Joanna, Justyna Gornowicz-Porowska, Jaromir Budzianowski, Gerard Nowak, Grzegorz Schroeder, and Joanna Kurczewska. 2022. "Medicinal Herbs in the Relief of Neurological, Cardiovascular, and Respiratory Symptoms after COVID-19 Infection A Literature Review" Cells 11, no. 12: 1897. https://doi.org/10.3390/cells11121897