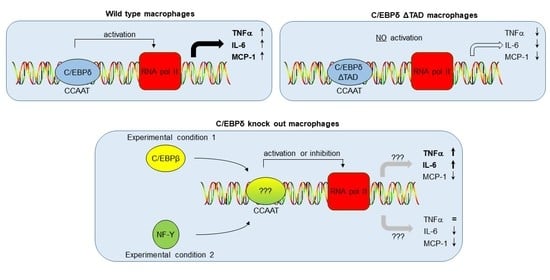
CEBPD Potentiates the Macrophage Inflammatory Response but CEBPD Knock-Out Macrophages Fail to Identify CEBPD-Dependent Pro-Inflammatory Transcriptional Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bone Marrow Derived Macrophage (BMDM) Isolation
2.3. Peritoneal Macrophage Isolation
2.4. Cell Stimulation Experiments
2.5. Cytokine Measurements
2.6. RNA Isolation, cDNA Synthesis and RT-qPCR
2.7. Mining of Publicly Available RNA Microarray Datasets
2.8. CRISPR/Cas9 Genome Editing
2.9. RAW264.7 Cell Culture and Stimulation
2.10. RNA Library Preparation and Sequencing
2.11. Bioinformatics Analysis of RNAseq
2.12. Statistical Analysis
3. Results
3.1. Context-Dependent Effect of C/EBPδ on LPS-Induced Cytokine Production
3.2. Macrophage Differentiation/Activation Modifies Expression Levels of Alternative CCAATT Box Binding Proteins
3.3. Diminished LPS-Induced Cytokine Production in C/EBPδ Transactivation Domain Mutant Macrophages
3.4. Discordant Transcriptional Programs in C/EBPδ Deficient and C/EBPδ Transactivation Domain Mutant Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lekstrom-Himes, J.; Xanthopoulos, K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 1998, 273, 28545–28548. [Google Scholar] [CrossRef] [Green Version]
- Pulido-Salgado, M.; Vidal-Taboada, J.M.; Saura, J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS. Prog. Neurobiol. 2015, 132, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365, 561–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, C.Y.; Chang, W.C.; Wang, J.M. Biological roles of CCAAT/Enhancer-binding protein delta during inflammation. J. Biomed. Sci. 2015, 22, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullmann, T.; Luckhardt, S.; Wolf, M.; Parnham, M.J.; Resch, E. High-Throughput Screening for CEBPD-Modulating Compounds in THP-1-Derived Reporter Macrophages Identifies Anti-Inflammatory HDAC and BET Inhibitors. Int. J. Mol. Sci. 2021, 22, 3022. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Ko, C.J.; Chen, L.C.; Wang, W.L.; Chang, W.C. Functional role of NF-IL6 beta and its sumoylation and acetylation modifications in promoter activation of cyclooxygenase 2 gene. Nucleic Acids Res. 2006, 34, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.K.; Cho, Y.H.; Lee, G.H.; Kim, S.G. Induction of cyclooxygenase-2 by bovine type I collagen in macrophages via C/EBP and CREB activation by multiple cell signaling pathways. Biochem. Pharmacol. 2004, 67, 2239–2250. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Berenbaum, F.; Humbert, L.; Bian, H.M.; Bereziat, G.; Crofford, L.; Olivier, J.L. Critical role of C/EBP delta and C/EBP beta factors in the stimulation of the cyclooxygenase-2 gene transcription by interleukin-1 beta in articular chondrocytes. Eur. J. Biochem. 2000, 267, 6798–6809. [Google Scholar] [CrossRef]
- Balamurugan, K.; Sharan, S.; Klarmann, K.D.; Zhang, Y.; Coppola, V.; Summers, G.H.; Roger, T.; Morrison, D.K.; Keller, J.R.; Sterneck, E. FBXW7alpha attenuates inflammatory signalling by downregulating C/EBPdelta and its target gene Tlr4. Nat. Commun. 2013, 4, 1662. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Zhu, M.; Staiger, J.; Johnson, P.F.; Gao, H. C5a-regulated CCAAT/Enhancer-binding proteins beta and delta are Essential in Fc gamma receptor-mediated inflammatory cytokine and chemokine production in macrophages. J. Biol. Chem. 2012, 287, 3217–3230. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Johnson, P.F.; Tang, H.; Ye, Y.; Wu, M.; Gao, H. CCAAT/Enhancer-binding protein delta is a critical mediator of lipopolysaccharide-induced acute lung injury. Am. J. Pathol. 2012, 182, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Liu, Y.; Gao, H.; Wang, X. Suppressors of cytokine signaling 3 is essential for FcγR-mediated inflammatory response via enhancing CCAAT/enhancer-binding protein δ transcriptional activity in macrophages. Exp. Cell Res. 2015, 337, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Maitra, U.; Gan, L.; Chang, S.; Li, L. Low-dose endotoxin induces inflammation by selectively removing nuclear receptors and activating CCAAT/Enhancer-binding protein delta. J. Immunol. 2011, 186, 4467–4473. [Google Scholar] [CrossRef]
- Slofstra, S.H.; Groot, A.P.; Obdeijn, M.H.; Reitsma, P.H.; ten Cate, H.; Spek, C.A. Gene expression profiling identifies C/EBPδ as a candidate regulator of endotoxin-induced disseminated intravascular coagulation. Am. J. Resp. Crit. Care Med. 2007, 176, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-H.; Huang, H.-S.; Wu, P.-T.; Jou, I.-M.; Pan, M.-H.; Chang, W.-C.; Wang, D.D.H.; Wang, J.-M. Role of macrophage CCAAT/Enhancer binding protein delta in the pathogenesis of rheumatoid arthritis in collagen-induced arthritic mice. PLoS ONE 2012, 7, e45378. [Google Scholar] [CrossRef] [PubMed]
- Litvak, V.; Ramsey, S.A.; Rust, A.G.; Zak, D.E.; Kennedy, K.A.; Lampano, A.E.; Nykter, M.; Shmulevich, I.; Aderem, A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat. Immunol. 2009, 10, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Duitman, J.; Hoogendijk, A.J.; Groot, A.P.; Ruela, R.R.; van der Poll, T.; Florquin, S.; Spek, C.A. CCAAT-Enhancer-binding protein delta (C/EBPδ) protects against Klebsiella-pneumoniae-induced pulmonary infection; potential role for macrophage migration. J. Infect. Dis. 2012, 206, 1826–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.C.; Kim, I.; Lye, E.; Shen, F.; Suzuki, N.; Suzuki, S.; Gerondakis, S.; Akira, S.; Gaffen, S.L.; Yeh, W.C.; et al. Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines. J. Immunol. 2009, 182, 7212–7221. [Google Scholar] [CrossRef]
- Duitman, J.W.; Hoogendijk, A.J.; van der Poll, T.; Spek, C.A. CCAAT/enhancer-binding protein δ: Multifaceted regulator in respiratory disease. Am. J. Pathol. 2013, 182, 1459. [Google Scholar] [CrossRef]
- Ayoubi, T.A.; Creemers, J.W.; Roebroek, A.J.; Van de Ven, W.J. Expression of the dibasic proprotein processing enzyme furin is directed by multiple promoters. J. Biol. Chem. 1994, 269, 9298–9303. [Google Scholar] [CrossRef]
- Colangelo, A.M.; Johnson, P.F.; Mocchetti, I. beta-adrenergic receptor-induced activation of nerve growth factor gene transcription in rat cerebral cortex involves CCAAT/enhancer-binding protein delta. Proc. Natl. Acad. Sci. USA 1998, 95, 10920–10925. [Google Scholar] [CrossRef]
- Balamurugan, K.; Sterneck, E. The many faces of C/EBPδ and their relevance for inflammation and cancer. Int. J. Biol. Sci. 2013, 9, 917–933. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.M.; Baer, M.; Williams, S.C.; Johnson, P.F.; Schwartz, R.C. Redundancy of C/EBP alpha, -beta, and -delta in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J. Immunol. 1998, 160, 2334–2342. [Google Scholar]
- Sterneck, E.; Paylor, R.; Jackson-Lewis, V.; Libbey, M.; Przedborski, S.; Tessarollo, L.; Crawley, J.N.; Johnson, P.F. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc. Natl. Acad. Sci. USA 1998, 95, 10908–10913. [Google Scholar] [CrossRef]
- Dessing, M.C.; Knapp, S.; Florquin, S.; de Vos, A.F.; van der Poll, T. CD14 facilitates invasive respiratory tract infection by Streptococcus pneumoniae. Am. J. Respir. Crit. Care Med. 2007, 175, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Van den Boogaard, F.E.; Brands, X.; Duitman, J.; de Stoppelaar, S.F.; Borensztajn, K.S.; Roelofs, J.J.T.H.; Hollenberg, M.D.; Spek, C.A.; Schultz, M.J.; Veer, C.v.; et al. Protease-activated receptor 2 facilitates bacterial dissemination in pneumococcal pneumonia. J. Infect. Dis. 2018, 217, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Dis. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- R2: Genomics Analysis and Visualization Platform. Available online: http://r2.amc.nl (accessed on 24 November 2020).
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Poli, V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998, 273, 29279–29282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, R. A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res. 1998, 26, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Ly, L.L.; Yoshida, H.; Yamaguchi, M. Nuclear transcription factor Y and its roles in cellular processes related to human disease. Am. J. Cancer Res. 2013, 3, 339–346. [Google Scholar] [PubMed]
- Banerjee, S.; Xie, N.; Cui, H.; Tan, Z.; Yang, S.; Icyuz, M.; Abraham, E.; Liu, G. MicroRNA let-7c regulates macrophage polarization. J. Immunol. 2013, 190, 6542–6549. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Screpanti, I.; Romani, L.; Musiani, P.; Modesti, A.; Fattori, E.; Lazzaro, D.; Sellitto, C.; Scarpa, S.; Bellavia, D.; Lattanzio, G. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995, 14, 1932–1941. [Google Scholar] [CrossRef]
- Tanaka, T.; Akira, S.; Yoshida, K.; Umemoto, M.; Yoneda, Y.; Shirafuji, N.; Fujiwara, H.; Suematsu, S.; Yoshida, N.; Kishimoto, T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 1995, 80, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Gorgoni, B.; Maritano, D.; Marthyn, P.; Righi, M.; Poli, V. C/EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mRNAs in macrophages. J. Immunol. 2002, 168, 4055–4062. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, S.; Liu, Y.; Nonnemacher, M.R.; Dampier, W.; Wigdahl, B. CCAAT enhancer binding protein and nuclear factor of activated T cells regulate HIV-1 LTR via a novel conserved downstream site in cells of the monocyte-macrophage lineage. PLoS ONE 2014, 9, e88116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orengo, C.A.; Thornton, J.M. Protein families and their evolution—A structural perspective. Annu. Rev. Biochem. 2005, 74, 867–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duitman, J.; Valls Serón, M.; Engelen-Lee, J.; Brouwer, M.C.; Spek, C.A.; van de Beek, D. Detrimental role for CCAAT/enhancer binding protein δ in blood-borne brain infection. BMC Infect. Dis. 2016, 16, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valls Serón, M.; Duitman, J.; Geldhoff, M.; Engelen-Lee, J.; Havik, S.R.; Brouwer, M.C.; van de Beek, D.; Spek, C.A. CCAAT/enhancer-binding protein δ (C/EBPδ) aggravates inflammation and bacterial dissemination during pneumococcal meningitis. J. Neuroinflammation 2015, 12, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duitman, J.; Schouten, M.; Groot, A.P.; Borensztajn, K.S.; Daalhuisen, J.B.; Florquin, S.; van der Poll, T.; Spek, C.A. CCAAT/enhancer-binding protein δ facilitates bacterial dissemination during pneumococcal pneumonia in a platelet-activating factor receptor-dependent manner. Proc. Natl. Acad. Sci. USA 2012, 109, 9113–9118. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Fu, Q.; Shah, S.K.; Melnyk, S.B.; Sterneck, E.; Hauer-Jensen, M.; Pawar, S.A. C/EBPδ protects from radiation-induced intestinal injury and sepsis by suppression of inflammatory and nitrosative stress. Sci. Rep. 2019, 9, 13953. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.A.; Shao, L.; Chang, J.; Wang, W.; Pathak, R.; Zhu, X.; Wang, J.; Hendrickson, H.; Boerma, M.; Sterneck, E.; et al. C/EBPδ deficiency sensitizes mice to ionizing radiation-induced hematopoietic and intestinal injury. PLoS ONE 2014, 9, e94967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamurugan, K.; Wang, J.M.; Tsai, H.H.; Sharan, S.; Anver, M.; Leighty, R.; Sterneck, E. The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 2010, 29, 4106–4117. [Google Scholar] [CrossRef] [Green Version]
- Duitman, J.; Borensztajn, K.S.; Pulskens, W.P.; Leemans, J.C.; Florquin, S.; Spek, C.A. CCAAT-enhancer binding protein delta (C/EBPδ) attenuates tubular injury and tubulointerstitial fibrogenesis during chronic obstructive nephropathy. Lab. Investig. 2014, 94, 89–97. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spek, C.A.; Aberson, H.L.; Butler, J.M.; de Vos, A.F.; Duitman, J. CEBPD Potentiates the Macrophage Inflammatory Response but CEBPD Knock-Out Macrophages Fail to Identify CEBPD-Dependent Pro-Inflammatory Transcriptional Programs. Cells 2021, 10, 2233. https://doi.org/10.3390/cells10092233
Spek CA, Aberson HL, Butler JM, de Vos AF, Duitman J. CEBPD Potentiates the Macrophage Inflammatory Response but CEBPD Knock-Out Macrophages Fail to Identify CEBPD-Dependent Pro-Inflammatory Transcriptional Programs. Cells. 2021; 10(9):2233. https://doi.org/10.3390/cells10092233
Chicago/Turabian StyleSpek, C. Arnold, Hella L. Aberson, Joe M. Butler, Alex F. de Vos, and JanWillem Duitman. 2021. "CEBPD Potentiates the Macrophage Inflammatory Response but CEBPD Knock-Out Macrophages Fail to Identify CEBPD-Dependent Pro-Inflammatory Transcriptional Programs" Cells 10, no. 9: 2233. https://doi.org/10.3390/cells10092233