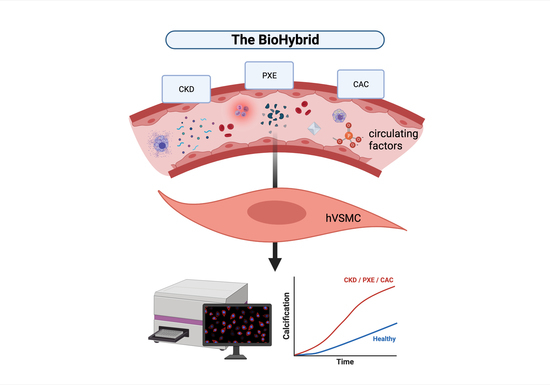
Development of the BioHybrid Assay: Combining Primary Human Vascular Smooth Muscle Cells and Blood to Measure Vascular Calcification Propensity
Abstract
:1. Introduction
2. Materials and Methods
2.1. hVSMC Culturing and Characterisation
2.2. BioHybrid Assay
2.3. o-Cresolphthalein Assay
2.4. T50 Assay
2.5. Serum and Plasma Preparation
2.6. Dephosphorylated-Uncarboxylated Matrix Gla Protein (dp-ucMGP) Measurement
2.7. Data Analysis and Statistics
3. Results
3.1. Development of the BioHybrid Calcification Assay
3.2. Calcification Propensity of CKD5D Serum
3.3. Calcification Propensity in Cohorts of Chronic Kidney Disease Patients, Pseudoxanthoma Elasticum Patients and Patients with Coronary Artery Calcification as Compared to Control
3.4. Calcification Propensity of Serum from Aortic Valve Calcification Patients with Vitamin K1 Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Risk Factor Collaborators Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [CrossRef] [Green Version]
- Khambhati, J.; Allard-Ratick, M.; Dhindsa, D.; Lee, S.; Chen, J.; Sandesara, P.B.; O’Neal, W.; Quyyumi, A.A.; Wong, N.D.; Blumenthal, R.S.; et al. The Art of Cardiovascular Risk Assessment. Clin. Cardiol. 2018, 41, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Schurgers, L.J.; Akbulut, A.C.; Kaczor, D.M.; Halder, M.; Koenen, R.R.; Kramann, R. Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles. Front. Cardiovasc. Med. 2018, 5, 36. [Google Scholar] [CrossRef]
- Rennenberg, R.J.M.W.; Kessels, A.G.H.; Schurgers, L.J.; van Engelshoven, J.M.A.; de Leeuw, P.W.; Kroon, A.A. Vascular Calcifications as a Marker of Increased Cardiovascular Risk: A Meta-Analysis. Vasc. Health Risk Manag. 2009, 5, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Raggi, P.; Callister, T.Q.; Shaw, L.J. Progression of Coronary Artery Calcium and Risk of First Myocardial Infarction in Patients Receiving Cholesterol-Lowering Therapy. Arter. Thromb Vasc. Biol. 2004, 24, 1272–1277. [Google Scholar] [CrossRef]
- Pasch, A.; Farese, S.; Gräber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-Based Test Measures Overall Propensity for Calcification in Serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Krüger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower Progress of Aortic Valve Calcification with Vitamin K Supplementation: Results From a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla Protein Is an Independent Predictor of Both Intimal and Medial Vascular Calcification in Chronic Kidney Disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human Vascular Smooth Muscle Cells Undergo Vesicle-Mediated Calcification in Response to Changes in Extracellular Calcium and Phosphate Concentrations: A Potential Mechanism for Accelerated Vascular Calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A Regulation of Calcified Matrix Metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef]
- Pasch, A.; Block, G.A.; Bachtler, M.; Smith, E.R.; Jahnen-Dechent, W.; Arampatzis, S.; Chertow, G.M.; Parfrey, P.; Ma, X.; Floege, J. Blood Calcification Propensity, Cardiovascular Events, and Survival in Patients Receiving Hemodialysis in the EVOLVE Trial. Clin. J. Am. Soc. Nephrol. 2017, 12, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum Calcification Propensity Predicts All-Cause Mortality in Predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Keyzer, C.A.; de Borst, M.H.; van den Berg, E.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.L.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016, 27, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahle, D.O.; Åsberg, A.; Hartmann, A.; Holdaas, H.; Bachtler, M.; Jenssen, T.G.; Dionisi, M.; Pasch, A. Serum Calcification Propensity Is a Strong and Independent Determinant of Cardiac and All-Cause Mortality in Kidney Transplant Recipients. Am. J. Transpl. 2016, 16, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitto, J.; Li, Q.; Jiang, Q. Pseudoxanthoma Elasticum: Molecular Genetics and Putative Pathomechanisms. J. Investig. Derm. 2010, 130, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitto, J.; Li, Q.; van de Wetering, K.; Váradi, A.; Terry, S.F. Insights into Pathomechanisms and Treatment Development in Heritable Ectopic Mineralization Disorders: Summary of the PXE International Biennial Research Symposium-2016. J. Investig. Derm. 2017, 137, 790–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shaughnessy, M.M.; Liu, S.; Montez-Rath, M.E.; Lafayette, R.A.; Winkelmayer, W.C. Cause of Kidney Disease and Cardiovascular Events in a National Cohort of US Patients with End-Stage Renal Disease on Dialysis: A Retrospective Analysis. Eur. Heart J. 2019, 40, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Akiba, T.; Suzuki, K.; Uchida, K.; Ogawa, T.; Majima, K.; Watanabe, R.; Aoki, T.; Nihei, H. Assessment of Coronary Artery Calcification in Hemodialysis Patients Using Multi-Detector Spiral CT Scan. Hypertens. Res. 2004, 27, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-R.; Zhang, J.-J.; Xu, X.-X.; Wu, Y.-G. Prevalence of Coronary Artery Calcification and Its Association with Mortality, Cardiovascular Events in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Ren. Fail. 2019, 41, 244–256. [Google Scholar] [CrossRef]
- Raggi, P. Cardiovascular Disease: Coronary Artery Calcification Predicts Risk of CVD in Patients with CKD. Nat. Rev. Nephrol. 2017, 13, 324–326. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kitagawa, T.; Kihara, Y. Clinical Implications of the Coronary Artery Calcium Score in Japanese Patients. J. Atheroscler. Thromb. 2014, 21, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, D.; Corrao, S.; Battaglia, Y.; Andreucci, M.; Caiazza, A.; Carlomagno, A.; Lamberti, M.; Pezone, N.; Pota, A.; Russo, L.; et al. Progression of Coronary Artery Calcification and Cardiac Events in Patients with Chronic Renal Disease Not Receiving Dialysis. Kidney Int. 2011, 80, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavroulopoulos, A.; Porter, C.J.; Pointon, K.; Monaghan, J.M.; Roe, S.D.; Cassidy, M.J.D. Evolution of Coronary Artery Calcification in Patients with Chronic Kidney Disease Stages 3 and 4, with and without Diabetes. Nephrol. Dial. Transpl. 2011, 26, 2582–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarche, M.C.; Hopman, W.M.; Garland, J.S.; White, C.A.; Holden, R.M. Relationship of Coronary Artery Calcification with Renal Function Decline and Mortality in Predialysis Chronic Kidney Disease Patients. Nephrol. Dial. Transpl. 2019, 34, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Gheduzzi, D.; Boraldi, F.; Annovi, G.; DeVincenzi, C.P.; Schurgers, L.J.; Vermeer, C.; Quaglino, D.; Ronchetti, I.P. Matrix Gla Protein Is Involved in Elastic Fiber Calcification in the Dermis of Pseudoxanthoma Elasticum Patients. Lab. Investig. 2007, 87, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaminon, A.M.G.; Akbulut, A.C.; Rapp, N.; Kramann, R.; Biessen, E.A.L.; Temmerman, L.; Mees, B.; Brandenburg, V.; Dzhanaev, R.; Jahnen-Dechent, W.; et al. Development of the BioHybrid Assay: Combining Primary Human Vascular Smooth Muscle Cells and Blood to Measure Vascular Calcification Propensity. Cells 2021, 10, 2097. https://doi.org/10.3390/cells10082097
Jaminon AMG, Akbulut AC, Rapp N, Kramann R, Biessen EAL, Temmerman L, Mees B, Brandenburg V, Dzhanaev R, Jahnen-Dechent W, et al. Development of the BioHybrid Assay: Combining Primary Human Vascular Smooth Muscle Cells and Blood to Measure Vascular Calcification Propensity. Cells. 2021; 10(8):2097. https://doi.org/10.3390/cells10082097
Chicago/Turabian StyleJaminon, Armand M. G., Asim C. Akbulut, Niko Rapp, Rafael Kramann, Erik A. L. Biessen, Lieve Temmerman, Barend Mees, Vincent Brandenburg, Robert Dzhanaev, Willi Jahnen-Dechent, and et al. 2021. "Development of the BioHybrid Assay: Combining Primary Human Vascular Smooth Muscle Cells and Blood to Measure Vascular Calcification Propensity" Cells 10, no. 8: 2097. https://doi.org/10.3390/cells10082097