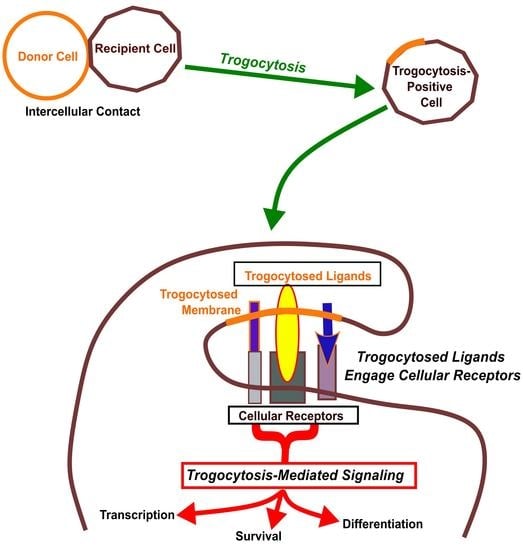
Lymphocytes and Trogocytosis-Mediated Signaling
Abstract
:1. Trogocytosis
2. Mechanism of Trogocytosis
3. Current Model of Trogocytosis
4. Trogocytosis-Positive Cells as Antigen Presenting Cells
5. Membrane Topology of Trogocytosed Molecules
6. Trogocytosis-Mediated Signaling
| Effector Subset | Transcriptional Regulator | Characteristic Cytokine | Immunological Functions |
|---|---|---|---|
| TH1 [132,133] | T-bet [134] | IFNγ | Intracellular pathogens and cancer |
| TH2 [132,133] | Gata-3 [135] | IL-4 | Helminthes and parasites |
| TH17 [136,137] | RORγt [138] | IL-17 | Extracellular pathogens, neutrophil activation, inflammation |
| TFH [139,140,141] | Bcl6 [142] | IL-21 | Mediate germinal center reaction (e.g., somatic hypermutation) |
| TH22 [143] | AhR [144,145] | IL-22 | Extracellular bacteria, skin protection |
| TReg [146,147] | Foxp3 [148,149] | TGF-β | Regulate immune responses |
7. Potential Role of Trogocytosis-Mediated Signaling in CD4+ Cell Differentiation: TFH
8. Potential Role of Trogocytosis-Mediated Signaling in CD4+ Memory Cell Differentiation
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Brown, T. Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J. Med. Microbiol. 1979, 12, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, E.; Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003, 4, 815. [Google Scholar] [CrossRef]
- Osborne, D.G.; Wetzel, S.A. Trogocytosis Results in Sustained Intracellular Signaling in CD4+ T Cells. J. Immunol. 2012, 189, 4728–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzel, S.A.; Parker, D.C. MHC Transfer from APC to T Cells Following Antigen Recognition. Crit. Rev. Immunol. 2006, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Osborne, D.G.; Browning, D.L.; Parker, D.C.; Wetzel, S.A. Anergic CD4+ T cells form mature immunological synapses with enhanced accumulation of c-Cbl and Cbl-b. J. Immunol. 2010, 184, 3598–3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzel, S.A.; McKeithan, T.W.; Parker, D.C. Peptide-Specific Intercellular Transfer of MHC Class II to CD4+ T Cells Directly from the Immunological Synapse upon Cellular Dissociation. J. Immunol. 2005, 174, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, E.; Takahashi, Y.; Lichtenfeld, J.; Tanaka, R.; Yoshida, A.; Sugamura, K.; Yamamoto, N.; Tanaka, Y. Functional CD4 T cells after intercellular molecular transfer of 0X40 ligand. J. Immunol. 2001, 167, 875–883. [Google Scholar] [CrossRef]
- Shi, M.; Hao, S.; Chan, T.; Xiang, J. CD4+ T cells stimulate memory CD8+ T cell expansion via acquired pMHC I complexes and costimulatory molecules, and IL-2 secretion. J. Leukoc. Biol. 2006, 80, 1354–1363. [Google Scholar] [CrossRef]
- Hudrisier, D.; Aucher, A.; Puaux, A.L.; Bordier, C.; Joly, E. Capture of Target Cell Membrane Components via Trogocytosis Is Triggered by a Selected Set of Surface Molecules on T or B Cells. J. Immunol. 2007, 178, 3637–3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamopoulou, E.; Diekmann, J.; Tolosa, E.; Kuntz, G.; Einsele, H.; Rammensee, H.G.; Topp, M.S. Human CD4+ T cells displaying viral epitopes elicit a functional virus-specific memory CD8+ T cell response. J. Immunol. 2007, 178, 5465–5472. [Google Scholar] [CrossRef] [Green Version]
- Umeshappa, C.S.; Huang, H.; Xie, Y.; Wei, Y.; Mulligan, S.J.; Deng, Y.; Xiang, J. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J. Immunol. 2009, 182, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Hudrisier, D.; Riond, J.; Mazarguil, H.; Gairin, J.E.; Joly, E. Cutting Edge: CTLs Rapidly Capture Membrane Fragments from Target Cells in a TCR Signaling-Dependent Manner. J. Immunol. 2001, 166, 3645–3649. [Google Scholar] [CrossRef] [Green Version]
- Riond, J.; Elhmouzi, J.; Hudrisier, D.; Gairin, J.E. Capture of membrane components via trogocytosis occurs in vivo during both dendritic cells and target cells encounter by CD8+ T cells. Scand. J. Immunol. 2007, 66, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.; Voelkl, S.; Palmisano, R.; Ullrich, E.; Bosch, J.J.; Mackensen, A. Antigen-Specific Transfer of Functional Programmed Death Ligand 1 from Human APCs onto CD8+ T Cells via Trogocytosis. J. Immunol. 2012, 188, 744–752. [Google Scholar] [CrossRef] [Green Version]
- Uzana, R.; Eisenberg, G.; Sagi, Y.; Frankenburg, S.; Merims, S.; Amariglio, N.; Yefenof, E.; Peretz, T.; Machlenkin, A.; Lotem, M. Trogocytosis Is a Gateway to Characterize Functional Diversity in Melanoma-Specific CD8+ T Cell Clones. J. Immunol. 2012, 188, 632–640. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, E.; Tabiasco, J.; Hudrisier, D.; Fournie, J.J. Synaptic transfer by human gamma delta T cells stimulated with soluble or cellular antigens. J. Immunol. 2002, 168, 6336–6343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, F.D.; Iber, D.; Neuberger, M.S. B cells acquire antigen from target cells after synapse formation. Nature 2001, 411, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Aucher, A.; Magdeleine, E.; Joly, E.; Hudrisier, D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood 2008, 111, 5621–5628. [Google Scholar] [CrossRef] [Green Version]
- Gardell, J.L.; Parker, D.C. CD40L is transferred to antigen-presenting B cells during delivery of T-cell help. Eur. J. Immunol. 2017, 47, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Poupot, M.; Fournié, J.-J.; Poupot, R. Trogocytosis and killing of IL-4-polarized monocytes by autologous NK cells. J. Leukoc. Biol. 2008, 84, 1298–1305. [Google Scholar] [CrossRef]
- Nakayama, M.; Takeda, K.; Kawano, M.; Takai, T.; Ishii, N.; Ogasawara, K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 18360–18365. [Google Scholar] [CrossRef] [Green Version]
- Miner, C.A.; Giri, T.K.; Meyer, C.E.; Shabsovich, M.; Tripathy, S.K. Acquisition of Activation Receptor Ligand by Trogocytosis Renders NK Cells Hyporesponsive. J. Immunol. 2015, 194, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Shiozawa, N.; Nagao, T.; Yoshikawa, S.; Yamanishi, Y.; Karasuyama, H. Trogocytosis of peptide–MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl. Acad. Sci. USA 2017, 114, 1111–1116. [Google Scholar] [CrossRef] [Green Version]
- Daubeuf, S.; Lindorfer, M.A.; Taylor, R.P.; Joly, E.; Hudrisier, D. The Direction of Plasma Membrane Exchange between Lymphocytes and Accessory Cells by Trogocytosis Is Influenced by the Nature of the Accessory Cell. J. Immunol. 2010, 184, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, A.K.; Doan-Xuan, Q.M.; Bacso, Z.; Csomós, I.; Balajthy, Z.; Fesus, L. Interaction of differentiated human adipocytes with macrophages leads to trogocytosis and selective IL-6 secretion. Cell Death Dis. 2015, 6, e1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.J.; Wu, C.H.; Shen, C.Y.; Kuo, Y.M.; Yu, C.L.; Hsieh, S.C. Membrane Transfer from Mononuclear Cells to Polymorphonuclear Neutrophils Transduces Cell Survival and Activation Signals in the Recipient Cells via Anti-Extrinsic Apoptotic and MAP Kinase Signaling Pathways. PLoS ONE 2016, 11, e0156262. [Google Scholar] [CrossRef] [Green Version]
- Valgardsdottir, R.; Cattaneo, I.; Klein, C.; Introna, M.; Figliuzzi, M.; Golay, J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 2017, 129, 2636–2644. [Google Scholar] [CrossRef] [Green Version]
- Mercer, F.; Ng, S.H.; Brown, T.M.; Boatman, G.; Johnson, P.J. Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol. 2018, 16, e2003885. [Google Scholar] [CrossRef]
- Zhang, Q.-J.; Li, X.-L.; Wang, D.; Huang, X.-C.; Mathis, J.M.; Duan, W.-M.; Knight, D.; Shi, R.; Glass, J.; Zhang, D.-Q.; et al. Trogocytosis of MHC-I/Peptide Complexes Derived from Tumors and Infected Cells Enhances Dendritic Cell Cross-Priming and Promotes Adaptive T Cell Responses. PLoS ONE 2008, 3, e3097. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Morandi, B.; Antsiferova, O.; Costa, G.; Oliveri, D.; Conte, R.; Pezzino, G.; Vermiglio, G.; Anastasi, G.P.; Navarra, G.; et al. Membrane Transfer from Tumor Cells Overcomes Deficient Phagocytic Ability of Plasmacytoid Dendritic Cells for the Acquisition and Presentation of Tumor Antigens. J. Immunol. 2014, 192, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Dance, A. Core Concept: Cells nibble one another via the under-appreciated process of trogocytosis. Proc. Natl. Acad. Sci. USA 2019, 116, 17608–17610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinhard, L.; Di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.K.; Ruthazer, E.S. Microglial trogocytosis and the complement system regulate axonal pruning in vivo. eLife 2021, 10, 10. [Google Scholar] [CrossRef]
- Barraud-Lange, V.; Naud-Barriant, N.; Bomsel, M.; Wolf, J.P.; Ziyyat, A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J. 2007, 21, 3446–3449. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.I.; Perez, F. Localized Intercellular Transfer of Ephrin-As by Trans-endocytosis Enables Long-Term Signaling. Dev. Cell 2020, 52, 104–117. [Google Scholar] [CrossRef]
- Gong, J.; Gaitanos, T.N.; Luu, O.; Huang, Y.; Gaitanos, L.; Lindner, J.; Winklbauer, R.; Klein, R. Gulp1 controls Eph/ephrin trogocytosis and is important for cell rearrangements during development. J. Cell Biol. 2019, 218, 3455–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdu, Y.; Maniscalco, C.; Heddleston, J.M.; Chew, T.L.; Nance, J. Developmentally programmed germ cell remodelling by endodermal cell cannibalism. Nat. Cell Biol. 2016, 18, 1302–1310. [Google Scholar] [CrossRef]
- Lis, R.; Capdet, J.; Mirshahi, P.; Lacroix-Triki, M.; Dagonnet, F.; Klein, C.; Mirshahi, M.; Fournie, J.J.; Rafii, A.; Poupot, M. Oncologic trogocytosis with Hospicells induces the expression of N-cadherin by breast cancer cells. Int. J. Oncol. 2010, 37, 1453–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooyman, D.L.; Byrne, G.W.; McClellan, S.; Nielsen, D.; Tone, M.; Waldmann, H.; Coffman, T.M.; McCurry, K.R.; Platt, J.L.; Logan, J.S. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science 1995, 269, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.A.; Jack, R.M.; Furlong, S.T. Lipid and membrane protein transfer from human neutrophils to schistosomes is mediated by ligand binding. J. Cell Sci. 1993, 106, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Furlong, S.T.; Thibault, K.S.; Rogers, R.A. Fluorescent phospholipids preferentially accumulate in sub-tegumental cells of schistosomula of Schistosoma mansoni. J. Cell Sci. 1992, 103, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukhopadhyay, A.; Andriani, G.; Machado, F.S.; Ashton, A.W.; Huang, H.; Weiss, L.M.; Tanowitz, H.B. Trypanosoma cruzi invasion is associated with trogocytosis. Microbes Infect. 2015, 17, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Miller, H.W.; Suleiman, R.L.; Ralston, K.S. Trogocytosis by Entamoeba histolytica Mediates Acquisition and Display of Human Cell Membrane Proteins and Evasion of Lysis by Human Serum. mBio 2019, 10, e00068-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, S.; Radlinski, L.; Taft-Benz, S.; Brunton, J.; Kawula, T.H. Trogocytosis-associated cell to cell spread of intracellular bacterial pathogens. eLife 2016, 5, e10625. [Google Scholar] [CrossRef] [PubMed]
- Rosenits, K.; Keppler, S.J.; Vucikuja, S.; Aichele, P. T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur. J. Immunol. 2010, 40, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.; Mix, D.; Reilly, A.A.; Yang Ye, X.; Winslow, G.M. Intercellular Transfer of a Soluble Viral Superantigen. J. Virol. 2000, 74, 8262–8267. [Google Scholar] [CrossRef] [Green Version]
- Cone, R.E.; Sprent, J.; Marchalonis, J.J. Antigen-Binding Specificity of Isolated Cell-Surface Immunoglobulin from Thymus Cells Activated to Histocompatibility Antigens. Proc. Natl. Acad. Sci. USA 1972, 69, 2556–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, L.; Sprent, J.; Miller, J.F.; Playfair, J.H. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature 1974, 251, 60–62. [Google Scholar] [CrossRef]
- Nagy, Z.; Elliott, B.E.; Nabholz, M.; Krammer, P.H.; Pernis, B. Specific binding of alloantigens to T cells activated in the mixed lymphocyte reaction. J. Exp. Med. 1976, 143, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Hudson, L.; Sprent, J. Specific adsorption of IgM antibody onto H-2-activated mouse T lymphocytes. J. Exp. Med. 1976, 143, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Benoist, C.; Mathis, D. Regulation of Major Histocompatibility Complex Class-II Genes: X, Y and Other Letters of the Alphabet. Annu. Rev. Immunol. 1990, 8, 681–715. [Google Scholar] [CrossRef]
- Bona, C.; Robineaux, R.; Anteunis, A.; Heuclin, C.; Astesano, A. Transfer of antigen from macrophages to lymphocytes. II. Immunological significance of the transfer of lipopolysaccharide. Immunology 1973, 24, 831–840. [Google Scholar] [PubMed]
- Nepom, J.T.; Benacerraf, B.; Germain, R.N. Acquisition of syngeneic I-A determinants by T cells proliferating in response to poly (Gly60Ala30, Tyr10). J. Immunol. 1981, 127, 888–892. [Google Scholar] [PubMed]
- Lorber, M.I.; Loken, M.R.; Stall, A.M.; Fitch, F.W. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J. Immunol. 1982, 128, 2798–2803. [Google Scholar] [PubMed]
- Germain, R.N.; Mayer, S.V.; Mescher, M.F. Role of I-region gene products in T cell activation. I. Stimulation of T lymphocyte proliferative responses by subcellular membrane preparations containing Ia alloantigens. J. Immunol. 1982, 128, 506–511. [Google Scholar] [PubMed]
- Sharrow, S.O.; Mathieson, B.J.; Singer, A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J. Immunol. 1981, 126, 1327–1335. [Google Scholar]
- Akkaya, B.; Oya, Y.; Akkaya, M.; Al Souz, J.; Holstein, A.H.; Kamenyeva, O.; Kabat, J.; Matsumura, R.; Dorward, D.W.; Glass, D.D.; et al. Regulatory T cells mediate specific suppression by depleting peptide–MHC class II from dendritic cells. Nat. Immunol. 2019, 20, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Bethune, M.T.; Wong, S.; Joglekar, A.V.; Leonard, M.T.; Wang, J.K.; Kim, J.T.; Cheng, D.; Peng, S.; Zaretsky, J.M.; et al. T cell antigen discovery via trogocytosis. Nat. Methods 2019, 16, 183–190. [Google Scholar] [CrossRef]
- Reed, J.; Wetzel, S.A. Trogocytosis-Mediated Intracellular Signaling in CD4+ T Cells Drives TH2-Associated Effector Cytokine Production and Differentiation. J. Immunol. 2019, 202, 2873–2887. [Google Scholar] [CrossRef]
- Tabiasco, J.; Espinosa, E.; Hudrisier, D.; Joly, E.; Fournie, J.J.; Vercellone, A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur. J. Immunol. 2002, 32, 1502–1508. [Google Scholar] [CrossRef]
- Tabiasco, J.; Vercellone, A.; Meggetto, F.; Hudrisier, D.; Brousset, P.; Fournié, J.J. Acquisition of Viral Receptor by NK Cells Through Immunological Synapse. J. Immunol. 2003, 170, 5993–5998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda-Navarro, P.; Reyburn, H.T. The Traffic of the NKG2D/Dap10 Receptor Complex during Natural Killer (NK) Cell Activation. J. Biol. Chem. 2009, 284, 16463–16472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.S.; Collinson, L.M.; Brzostek, J.; Eissmann, P.; Almeida, C.R.; McCann, F.E.; Burshtyn, D.; Davis, D.M. Membranous Structures Transfer Cell Surface Proteins Across NK Cell Immune Synapses. Traffic 2007, 8, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Carlin, L.M.; Eleme, K.; McCann, F.E.; Davis, D.M. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J. Exp. Med. 2001, 194, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Vanherberghen, B.; Andersson, K.; Carlin, L.M.; Nolte-’t Hoen, E.N.; Williams, G.S.; Hoglund, P.; Davis, D.M. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16873–16878. [Google Scholar] [CrossRef] [Green Version]
- Sabzevari, H.; Kantor, J.; Jaigirdar, A.; Tagaya, Y.; Naramura, M.; Hodge, J.; Bernon, J.; Schlom, J. Acquisition of CD80 (B7-1) by T Cells. J. Immunol. 2001, 166, 2505–2513. [Google Scholar] [CrossRef] [Green Version]
- Tatari-Calderone, Z.; Semnani, R.T.; Nutman, T.B.; Schlom, J.; Sabzevari, H. Acquisition of CD80 by Human T Cells at Early Stages of Activation: Functional Involvement of CD80 Acquisition in T Cell to T Cell Interaction. J. Immunol. 2002, 169, 6162–6169. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Huang, J.-F.; Kishimoto, H.; Brunmark, A.; Peterson, P.A.; Jackson, M.R.; Surh, C.D.; Cai, Z.; Sprent, J. T Cells Can Use Either T Cell Receptor or Cd28 Receptors to Absorb and Internalize Cell Surface Molecules Derived from Antigen-Presenting Cells. J. Exp. Med. 2000, 191, 1137–1148. [Google Scholar] [CrossRef]
- Caumartin, J.; LeMaoult, J.; Carosella, E.D. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl. Immunol. 2006, 17, 20–22. [Google Scholar] [CrossRef]
- Brown, R.; Kabani, K.; Favaloro, J.; Yang, S.; Ho, P.J.; Gibson, J.; Fromm, P.; Suen, H.; Woodland, N.; Nassif, N.; et al. CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood 2012, 120, 2055–2063. [Google Scholar] [CrossRef] [Green Version]
- Daubeuf, S.; Aucher, A.; Bordier, C.; Salles, A.; Serre, L.; Gaibelet, G.; Faye, J.-C.; Favre, G.; Joly, E.; Hudrisier, D. Preferential Transfer of Certain Plasma Membrane Proteins onto T and B Cells by Trogocytosis. PLoS ONE 2010, 5, e8716. [Google Scholar] [CrossRef] [Green Version]
- Stinchcombe, J.C.; Bossi, G.; Booth, S.; Griffiths, G.M. The Immunological Synapse of CTL Contains a Secretory Domain and Membrane Bridges. Immunity 2001, 15, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.M.; Dudek, R.W.; Mannie, M.D. Intercellular Exchange of Class II MHC Complexes: Ultrastructural Localization and Functional Presentation of Adsorbed I-A/Peptide Complexes. Cell. Immunol. 2001, 214, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Huang, H.; Liu, Y. A New Dynamic Model of CD8+ T Effector Cell Responses via CD4+ T Helper-Antigen-Presenting Cells. J. Immunol. 2005, 174, 7497–7505. [Google Scholar] [CrossRef] [Green Version]
- Boccasavia, V.L.; Bovolenta, E.R.; Villanueva, A.; Borroto, A.; Oeste, C.L.; van Santen, H.M.; Prieto, C.; Alonso-López, D.; Diaz-Muñoz, M.D.; Batista, F.D.; et al. Antigen presentation between T cells drives Th17 polarization under conditions of limiting antigen. Cell Rep. 2021, 34, 108861. [Google Scholar] [CrossRef] [PubMed]
- Puaux, A.-L.; Campanaud, J.; Salles, A.; Préville, X.; Timmerman, B.; Joly, E.; Hudrisier, D. A very rapid and simple assay based on trogocytosis to detect and measure specific T and B cell reactivity by flow cytometry. Eur. J. Immunol. 2006, 36, 779–788. [Google Scholar] [CrossRef]
- Chirkova, T.V.; Naykhin, A.N.; Petukhova, G.D.; Korenkov, D.A.; Donina, S.A.; Mironov, A.N.; Rudenko, L.G. Memory T-Cell Immune Response in Healthy Young Adults Vaccinated with Live Attenuated Influenza A (H5N2) Vaccine. Clin. Vaccine Immunol. 2011, 18, 1710–1718. [Google Scholar] [CrossRef] [Green Version]
- Beadling, C.; Slifka, M.K. Quantifying viable virus-specific T cells without a priori knowledge of fine epitope specificity. Nat. Med. 2006, 12, 1208–1212. [Google Scholar] [CrossRef]
- Somanchi, S.S.; Somanchi, A.; Cooper, L.J.; Lee, D.A. Engineering lymph node homing of ex vivo–expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood 2012, 119, 5164–5172. [Google Scholar] [CrossRef]
- Eisenberg, G.; Uzana, R.; Pato, A.; Frankenburg, S.; Merims, S.; Yefenof, E.; Ferrone, S.; Peretz, T.; Machlenkin, A.; Lotem, M. Imprinting of lymphocytes with melanoma antigens acquired by trogocytosis facilitates identification of tumor-reactive T cells. J. Immunol. 2013, 190, 5856–5865. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Nakayama, M.; Kawano, M.; Amagai, R.; Ishii, T.; Harigae, H.; Ogasawara, K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl. Acad. Sci. USA 2013, 110, 9421–9426. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.F.; Yang, Y.; Sepulveda, H.; Shi, W.; Hwang, I.; Peterson, P.A.; Jackson, M.R.; Sprent, J.; Cai, Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 1999, 286, 952–954. [Google Scholar] [CrossRef]
- Haastert, B.; Mellanby, R.J.; Anderton, S.M.; O’Connor, R.A. T Cells at the Site of Autoimmune Inflammation Show Increased Potential for Trogocytosis. PLoS ONE 2013, 8, e81404. [Google Scholar] [CrossRef] [PubMed]
- Hudrisier, D.; Riond, J.; Garidou, L.; Duthoit, C.; Joly, E. T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur. J. Immunol. 2005, 35, 2284–2294. [Google Scholar] [CrossRef]
- Hudrisier, D.; Bongrand, P. Intercellular transfer of antigen-presenting cell determinants onto T cells: Molecular mechanisms and biological significance. FASEB J. 2002, 16, 477–486. [Google Scholar] [CrossRef]
- Kedl, R.M.; Rees, W.A.; Hildeman, D.A.; Schaefer, B.; Mitchell, T.; Kappler, J.; Marrack, P. T Cells Compete for Access to Antigen-Bearing Antigen-Presenting Cells. J. Exp. Med. 2000, 192, 1105–1113. [Google Scholar] [CrossRef]
- Hwang, I.; Sprent, J. Role of actin cytoskeleton in T cell absorption and internalization of ligands from APC. J. Immunol. 2001, 166, 5099–5107. [Google Scholar] [CrossRef] [Green Version]
- Game, D.S.; Rogers, N.J.; Lechler, R.I. Acquisition of HLA-DR and Costimulatory Molecules by T Cells from Allogeneic Antigen Presenting Cells. Am. J. Transplant. 2005, 5, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Kongsomros, S.; Thanunchai, M.; Manopwisedjaroen, S.; Na-Ek, P.; Wang, S.F.; Taechalertpaisarn, T.; Thitithanyanont, A. Trogocytosis with monocytes associated with increased alpha2,3 sialic acid expression on B cells during H5N1 influenza virus infection. PLoS ONE 2020, 15, e0239488. [Google Scholar] [CrossRef] [PubMed]
- Daubeuf, S.; Bordier, C.; Hudrisier, D.; Joly, E. Suitability of various membrane lipophilic probes for the detection of trogocytosis by flow cytometry. Cytom. Part A 2009, 75, 380–389. [Google Scholar] [CrossRef]
- Rechavi, O.; Goldstein, I.; Vernitsky, H.; Rotblat, B.; Kloog, Y. Intercellular Transfer of Oncogenic H-Ras at the Immunological Synapse. PLoS ONE 2007, 2, e1204. [Google Scholar] [CrossRef]
- McCann, F.E.; Eissmann, P.; Önfelt, B.; Leung, R.; Davis, D.M. The Activating NKG2D Ligand MHC Class I-Related Chain A Transfers from Target Cells to NK Cells in a Manner That Allows Functional Consequences. J. Immunol. 2007, 178, 3418–3426. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, B.; Martínez-Martín, N. RRas2, RhoG and T-cell phagocytosis. Small GTPases 2012, 3, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martín, N.; Fernández-Arenas, E.; Cemerski, S.; Delgado, P.; Turner, M.; Heuser, J.; Irvine, D.J.; Huang, B.; Bustelo, X.R.; Shaw, A.; et al. T Cell Receptor Internalization from the Immunological Synapse Is Mediated by TC21 and RhoG GTPase-Dependent Phagocytosis. Immunity 2011, 35, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopfer, E.P.; Minguet, S.; Schamel, W.W. A New Vampire Saga: The Molecular Mechanism of T Cell Trogocytosis. Immunity 2011, 35, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Taams, L.S.; Van Eden, W.; Wauben, M.H. Antigen presentation by T cells versus professional antigen-presenting cells (APC): Differential consequences for T cell activation and subsequent T cell-APC interactions. Eur. J. Immunol. 1999, 29, 1543–1550. [Google Scholar] [CrossRef]
- Mannie, M.D.; Nardella, J.P.; White, G.A.; Arnold, P.Y.; Davidian, D.K. Class II MHC/Peptide Complexes on T Cell Antigen-Presenting Cells: Agonistic Antigen Recognition Inhibits Subsequent Antigen Presentation. Cell. Immunol. 1998, 186, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ding, Z.C.; Fu, J.; Levitsky, H.I. Presentation of Acquired Peptide-MHC Class II Ligands by CD4+ Regulatory T Cells or Helper Cells Differentially Regulates Antigen-Specific CD4+ T Cell Response. J. Immunol. 2011, 186, 2148–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, M. Antigen Presentation by MHC-Dressed Cells. Front. Immunol. 2014, 5, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helft, J.; Jacquet, A.; Joncker, N.T.; Grandjean, I.; Dorothee, G.; Kissenpfennig, A.; Malissen, B.; Matzinger, P.; Lantz, O. Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood 2008, 112, 1249–1258. [Google Scholar] [CrossRef]
- Dionne, S.O.; Smith, M.H.; Marincola, F.M.; Lake, D.F. Antigen Presentation of a Modified Tumor-Derived Peptide by Tumor Infiltrating Lymphocytes. Cell. Immunol. 2001, 214, 139–144. [Google Scholar] [CrossRef]
- Cox, J.H.; McMichael, A.J.; Screaton, G.R.; Xu, X.-N. CTLs target Th cells that acquire bystander MHC class I-peptide complex from APCs. J. Immunol. 2007, 179, 830–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhainaut, M.; Moser, M. Regulation of Immune Reactivity by Intercellular Transfer. Front. Immunol. 2014, 5, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegre, E.; Howangyin, K.Y.; Favier, B.; Baudhuin, J.; Lesport, E.; Daouya, M.; González, Á.; Carosella, E.D.; LeMaoult, J. Membrane redistributions through multi-intercellular exchanges and serial trogocytosis. Cell Res. 2010, 20, 1239–1251. [Google Scholar] [CrossRef]
- LeMaoult, J.; Caumartin, J.; Daouya, M.; Favier, B.; Le Rond, S.; Gonzalez, A.; Carosella, E.D. Immune regulation by pretenders: Cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 2007, 109, 2040–2048. [Google Scholar] [CrossRef]
- Gu, P.; Gao, J.F.; D’Souza, C.A.; Kowalczyk, A.; Chou, K.-Y.; Zhang, L. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell. Mol. Immunol. 2012, 9, 136–146. [Google Scholar] [CrossRef]
- Brown, R.; Suen, H.; Favaloro, J.; Yang, S.; Ho, P.J.; Gibson, J.; Joshua, D. Trogocytosis generates acquired regulatory T cells adding further complexity to the dysfunctional immune response in multiple myeloma. Oncoimmunology 2012, 1, 1658–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford McIntyre, M.S.; Young, K.J.; Gao, J.; Joe, B.; Zhang, L. Cutting edge: In vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J. Immunol. 2008, 181, 2271–2275. [Google Scholar] [CrossRef]
- Hao, S.; Yuan, J.; Xu, S.; Munegowda, M.A.; Deng, Y.; Gordon, J.; Xing, Z.; Xiang, J. Antigen specificity acquisition of adoptive CD4+ regulatory T cells via acquired peptide-MHC class I complexes. J. Immunol. 2008, 181, 2428–2437. [Google Scholar] [CrossRef] [Green Version]
- Bahcheli, D.; Hay, V.; Nadeau, J.L.; Piccirillo, C.A. Transfer of cell membrane components via trogocytosis occurs in CD4+ Foxp3+ CD25+ regulatory T-cell contact-dependent suppression. Autoimmunity 2011, 44, 607–615. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Joung, S.; Peek, M.J.; Nanan, R. Expansion of CD4+ HLA-G+ T Cell in Human Pregnancy is Impaired in Pre-eclampsia. Am. J. Reprod. Immunol. 2014, 71, 217–228. [Google Scholar] [CrossRef]
- Amiot, L.; Vu, N.; Samson, M. Biology of the immunomodulatory molecule HLA-G in human liver diseases. J. Hepatol. 2015, 62, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Rebmann, V.; LeMaoult, J.; Horn, P.A.; Carosella, E.D.; Alegre, E. The immunosuppressive molecule HLA-G and its clinical implications. Crit. Rev. Clin. Lab. Sci. 2012, 49, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Yan, W.H. Intercellular transfer of HLA-G: Its potential in cancer immunology. Clin. Transl. Immunol. 2019, 8, e1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.A.; Xiang, J. Mechanisms of cellular communication through intercellular protein transfer. J. Cell. Mol. Med. 2011, 15, 1458–1473. [Google Scholar] [CrossRef]
- Hamieh, M.; Dobrin, A.; Cabriolu, A.; Van Der Stegen, S.J.C.; Giavridis, T.; Mansilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568, 112–116. [Google Scholar] [CrossRef]
- Romagnoli, P.A.; Premenko-Lanier, M.F.; Loria, G.D.; Altman, J.D. CD8 T Cell Memory Recall Is Enhanced by Novel Direct Interactions with CD4 T Cells Enabled by MHC Class II Transferred from APCs. PLoS ONE 2013, 8, e56999. [Google Scholar] [CrossRef]
- Mostböck, S.; Catalfamo, M.; Tagaya, Y.; Schlom, J.; Sabzevari, H. Acquisition of antigen presentasome (APS), an MHC/costimulatory complex, is a checkpoint of memory T-cell homeostasis. Blood 2007, 109, 2488–2495. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.; Undale, A.H.; Kieper, W.C.; Block, M.S.; Pease, L.R.; Celis, E. Direct Cross-Priming by Th Lymphocytes Generates Memory Cytotoxic Responses. J. Immunol. 2005, 174, 3967–3977. [Google Scholar] [CrossRef] [Green Version]
- Nolte-’t Hoen, E.N.; Wagenaar-Hilbers, J.P.; Peters, P.J.; Gadella, B.M.; van Eden, W.; Wauben, M.H. Uptake of membrane molecules from T cells endows antigen-presenting cells with novel functional properties. Eur. J. Immunol. 2004, 34, 3115–3125. [Google Scholar] [CrossRef]
- Romagnoli, P.; Hudrisier, D.; Van Meerwijk, J.P. Molecular Signature of Recent Thymic Selection Events on Effector and Regulatory CD4+ T Lymphocytes. J. Immunol. 2005, 175, 5751–5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, M.; Hori, A.; Toyoura, S.; Yamaguchi, S.-I. Shaping of T Cell Functions by Trogocytosis. Cells 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Hudrisier, D.; Clemenceau, B.; Balor, S.; Daubeuf, S.; Magdeleine, E.; Daëron, M.; Bruhns, P.; Vié, H. Ligand Binding but Undetected Functional Response of FcR after Their Capture by T Cells via Trogocytosis. J. Immunol. 2009, 183, 6102–6113. [Google Scholar] [CrossRef] [Green Version]
- Marcenaro, E.; Cantoni, C.; Pesce, S.; Prato, C.; Pende, D.; Agaugué, S.; Moretta, L.; Moretta, A. Uptake of CCR7 and acquisition of migratory properties by human KIR+ NK cells interacting with monocyte-derived DC or EBV cell lines: Regulation by KIR/HLA-class I interaction. Blood 2009, 114, 4108–4116. [Google Scholar] [CrossRef]
- Mack, M.; Kleinschmidt, A.; Brühl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachý, J.; Stangassinger, M.; Erfle, V.; Schlöndorff, D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef]
- Nakashima, M.; Watanabe, M.; Uchimaru, K.; Horie, R. Trogocytosis of ligand-receptor complex and its intracellular transport in CD30 signalling. Biol. Cell 2018, 110, 109–124. [Google Scholar] [CrossRef]
- Roda-Navarro, P.; Vales-Gomez, M.; Chisholm, S.E.; Reyburn, H.T. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc. Natl. Acad. Sci. USA 2006, 103, 11258–11263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Merwe, P.A.; Davis, S.J.; Shaw, A.S.; Dustin, M.L. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 2000, 12, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monks, C.R.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, K.; Mittrücker, H.-W.; Feger, U.; Welte, S.A.; Yokoyama, W.M.; Spies, T.; Rammensee, H.-G.; Steinle, A. Systemic NKG2D Down-Regulation Impairs NK and CD8 T Cell Responses In Vivo. J. Immunol. 2005, 175, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tagaya, Y.; Tolouei-Semnani, R.; Schlom, J.; Sabzevari, H. Physiological relevance of antigen presentasome (APS), an acquired MHC/costimulatory complex, in the sustained activation of CD4+ T cells in the absence of APCs. Blood 2005, 105, 3238–3246. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A Novel Transcription Factor, T-bet, Directs Th1 Lineage Commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Flavell, R.A. The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Harrington, L.E.; Mangan, P.R.; Gavrieli, M.; Murphy, K.M. Th17: An Effector CD4 T Cell Lineage with Regulatory T Cell Ties. Immunity 2006, 24, 677–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. Cxc Chemokine Receptor 5 Expression Defines Follicular Homing T Cells with B Cell Helper Function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Rott, L.S.; Clark-Lewis, I.; Campbell, D.J.; Wu, L.; Butcher, E.C. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 2001, 193, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Rao, S.; Tsai, L.M.; Lee, S.K.; He, Y.; Sutcliffe, E.L.; Srivastava, M.; Linterman, M.; Zheng, L.; Simpson, N.; et al. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity 2009, 31, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; O’Quinn, D.B.; Silberger, D.J.; Schoeb, T.R.; Fouser, L.; Ouyang, W.; Hatton, R.D.; Weaver, C.T. Th22 Cells Are an Important Source of IL-22 for Host Protection against Enteropathogenic Bacteria. Immunity 2012, 37, 1061–1075. [Google Scholar] [CrossRef] [Green Version]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.; Rubio, M.; Kenins, L.; Regairaz, C.; Woisetschlager, M.; Carballido, J.M.; Sarfati, M. The aryl hydrocarbon receptor (AhR) ligand VAF347 selectively acts on monocytes and naïve CD4+ Th cells to promote the development of IL-22-secreting Th cells. Hum. Immunol. 2012, 73, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Asano, M.; Toda, M.; Sakaguchi, N.; Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996, 184, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Fontenot, J.D.; Rasmussen, J.P.; Williams, L.M.; Dooley, J.L.; Farr, A.G.; Rudensky, A.Y. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 2005, 22, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Thauland, T.J.; Koguchi, Y.; Wetzel, S.A.; Dustin, M.L.; Parker, D.C. Th1 and Th2 cells form morphologically distinct immunological synapses. J. Immunol. 2008, 181, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, S.A.; McKeithan, T.W.; Parker, D.C. Live-cell dynamics and the role of costimulation in immunological synapse formation. J. Immunol. 2002, 169, 6092–6101. [Google Scholar] [CrossRef] [Green Version]
- Walden, P.R.; Eisen, H.N. Cognate peptides induce self-destruction of CD8+ cytolytic T lymphocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 9015–9019. [Google Scholar] [CrossRef] [Green Version]
- Ottenhoff, T.H.; Mutis, T. Specific killing of cytotoxic T cells and antigen-presenting cells by CD4+ cytotoxic T cell clones. A novel potentially immunoregulatory T-T cell interaction in man. J. Exp. Med. 1990, 171, 2011–2024. [Google Scholar] [CrossRef]
- Rachmilewitz, J.; Lanzavecchia, A. A temporal and spatial summation model for T-cell activation: Signal integration and antigen decoding. Trends Immunol. 2002, 23, 592–595. [Google Scholar] [CrossRef]
- Gunzer, M.; Schafer, A.; Borgmann, S.; Grabbe, S.; Zanker, K.S.; Brocker, E.B.; Kampgen, E.; Friedl, P. Antigen presentation in extracellular matrix: Interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity 2000, 13, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Underhill, D.M.; Bassetti, M.; Rudensky, A.; Aderem, A. Dynamic Interactions of Macrophages with T Cells during Antigen Presentation. J. Exp. Med. 1999, 190, 1909–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubo, N.J.; Jenkins, M.K. TCR signal quantity and quality in CD4+ T cell differentiation. Trends Immunol. 2014, 35, 591–596. [Google Scholar] [CrossRef]
- van Panhuys, N.; Klauschen, F.; Germain, R.N. T-Cell-Receptor-Dependent Signal Intensity Dominantly Controls CD4+ T Cell Polarization In Vivo. Immunity 2014, 41, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keck, S.; Schmaler, M.; Ganter, S.; Wyss, L.; Oberle, S.; Huseby, E.S.; Zehn, D.; King, C.G. Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 14852–14857. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Constant, S.; Jorritsma, P.; Bottomly, K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J. Immunol. 1997, 159, 5956–5963. [Google Scholar]
- Brogdon, J.L.; Leitenberg, D.; Bottomly, K. The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J. Immunol. 2002, 168, 3825–3832. [Google Scholar] [CrossRef] [Green Version]
- Constant, S.; Pfeiffer, C.; Woodard, A.; Pasqualini, T.; Bottomly, K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 1995, 182, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- van Panhuys, N. TCR Signal Strength Alters T–DC Activation and Interaction Times and Directs the Outcome of Differentiation. Front. Immunol. 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Leitenberg, D.; Boutin, Y.; Constant, S.; Bottomly, K. CD4 regulation of TCR signaling and T cell differentiation following stimulation with peptides of different affinities for the TCR. J. Immunol. 1998, 161, 1194–1203. [Google Scholar]
- Leitenberg, D.; Bottomly, K. Regulation of naive T cell differentiation by varying the potency of TCR signal transduction. Semin. Immunol. 1999, 11, 283–292. [Google Scholar] [CrossRef]
- Turner, M.S.; Isse, K.; Fischer, D.K.; Turnquist, H.R.; Morel, P.A. Low TCR signal strength induces combined expansion of Th2 and regulatory T cell populations that protect mice from the development of type 1 diabetes. Diabetologia 2014, 57, 1428–1436. [Google Scholar] [CrossRef]
- Linterman, M.A.; Vinuesa, C.G. Signals that influence T follicular helper cell differentiation and function. Semin. Immunopathol. 2010, 32, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Glatman Zaretsky, A.; Taylor, J.J.; King, I.L.; Marshall, F.A.; Mohrs, M.; Pearce, E.J. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 2009, 206, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Cullinan, P.; Sperling, A.I.; Burkhardt, J.K. The distal pole complex: A novel membrane domain distal to the immunological synapse. Immunol. Rev. 2002, 189, 111–122. [Google Scholar] [CrossRef]
- Allenspach, E.J.; Cullinan, P.; Tong, J.; Tang, Q.; Tesciuba, A.G.; Cannon, J.L.; Takahashi, S.M.; Morgan, R.; Burkhardt, J.K.; Sperling, A.I. ERM-Dependent Movement of CD43 Defines a Novel Protein Complex Distal to the Immunological Synapse. Immunity 2001, 15, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Reiner, S.L.; Sallusto, F.; Lanzavecchia, A. Division of Labor with a Workforce of One: Challenges in Specifying Effector and Memory T Cell Fate. Science 2007, 317, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Palanivel, V.R.; Kinjyo, I.; Schambach, F.; Intlekofer, A.M.; Banerjee, A.; Longworth, S.A.; Vinup, K.E.; Mrass, P.; Oliaro, J.; et al. Asymmetric T Lymphocyte Division in the Initiation of Adaptive Immune Responses. Science 2007, 315, 1687–1691. [Google Scholar] [CrossRef]
- Oliaro, J.; Van Ham, V.; Sacirbegovic, F.; Pasam, A.; Bomzon, Z.; Pham, K.; Ludford-Menting, M.J.; Waterhouse, N.J.; Bots, M.; Hawkins, E.D.; et al. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J. Immunol. 2010, 185, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iezzi, G.; Karjalainen, K.; Lanzavecchia, A. The Duration of Antigenic Stimulation Determines the Fate of Naive and Effector T Cells. Immunity 1998, 8, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Mempel, T.R.; Henrickson, S.E.; Von Adrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427, 154–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeithan, T.W. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl. Acad. Sci. USA 1995, 92, 5042–5046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrenas, J.; Wange, R.L.; Wang, J.L.; Isakov, N.; Samelson, L.E.; Germain, R.N. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science 1995, 267, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Kersh, B.E.; Kersh, G.J.; Allen, P.M. Partially phosphorylated T cell receptor zeta molecules can inhibit T cell activation. J. Exp. Med. 1999, 190, 1627–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reed, J.; Reichelt, M.; Wetzel, S.A. Lymphocytes and Trogocytosis-Mediated Signaling. Cells 2021, 10, 1478. https://doi.org/10.3390/cells10061478
Reed J, Reichelt M, Wetzel SA. Lymphocytes and Trogocytosis-Mediated Signaling. Cells. 2021; 10(6):1478. https://doi.org/10.3390/cells10061478
Chicago/Turabian StyleReed, Jim, Madison Reichelt, and Scott A. Wetzel. 2021. "Lymphocytes and Trogocytosis-Mediated Signaling" Cells 10, no. 6: 1478. https://doi.org/10.3390/cells10061478