Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses
Abstract
:1. VLCFA Structure, Biosynthesis and Distribution
1.1. VLCFA Definition and Chemical Diversity
1.2. VLCFA Biosynthesis
1.3. Arabidopsis KCS Multigenic Family
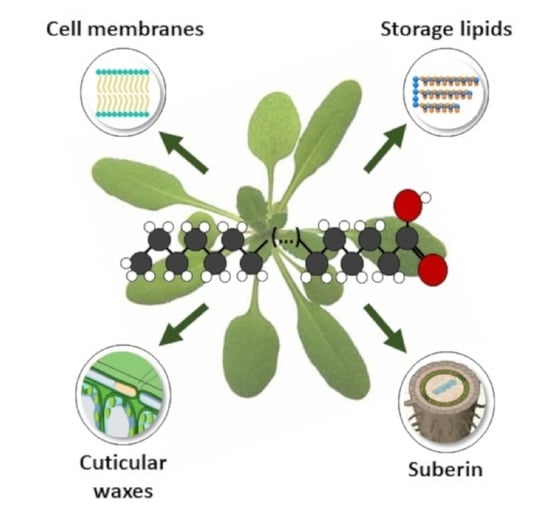
1.4. Lipids Incorporating VLCFAs
2. VLCFA-Derived Surface Lipids Constitute the Border between Plants and Its Surrounding Environment
2.1. Role of Cutin and Waxes in Aerial Plant Responses to Abiotic and Biotic Stresses
2.2. Role of Suberin in Plant Responses to Abiotic and Biotic Stresses
3. VLCFA and Sphingolipids in Plant Responses to Abiotic and Biotic Stresses
3.1. VLCFA in Plant Sphingolipids
3.2. Role of VLCFA-Sphingolipids in Structuring the Membranes
3.3. Role of Sphingolipids in Abiotic Stress Responses
3.4. Role of Sphingolipids in Biotic Stress Responses
4. VLCFA in Phosphatidylserine and in Plant Development
4.1. Phosphatidylserine Is Enriched in VLCFA
4.2. VLCFA and Plant Developmental Processes
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raffaele, S.; Leger, A.; Roby, D. Very Long Chain Fatty Acid and Lipid Signaling in the Response of Plants to Pathogens. Plant Signal. Behav. 2009, 4, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roudier, F.; Gissot, L.; Beaudoin, F.; Haslam, R.; Michaelson, L.; Marion, J.; Molino, D.; Lima, A.; Bach, L.; Morin, H.; et al. Very-Long-Chain Fatty Acids Are Involved in Polar Auxin Transport and Developmental Patterning in Arabidopsis. Plant Cell 2010, 22, 364–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobusawa, T.; Okushima, Y.; Nagata, N.; Kojima, M.; Sakakibara, H.; Umeda, M. Synthesis of Very-Long-Chain Fatty Acids in the Epidermis Controls Plant Organ Growth by Restricting Cell Proliferation. PLoS Biol. 2013, 11, e1001531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, D.-C.; Lavenus, J.; Goh, T.; Boutté, Y.; Drogue, Q.; Vaissayre, V.; Tellier, F.; Lucas, M.; Voß, U.; Gantet, P.; et al. PUCHI Regulates Very Long Chain Fatty Acid Biosynthesis during Lateral Root and Callus Formation. Proc. Natl. Acad. Sci. USA 2019, 116, 14325–14330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhukov, A.V.; Shumskaya, M. Very-Long-Chain Fatty Acids (VLCFAs) in Plant Response to Stress. Funct. Plant Biol. 2020, 47, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.; Faure, J.-D. Role of Very-Long-Chain Fatty Acids in Plant Development, When Chain Length Does Matter. C. R. Biol. 2010, 333, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Haslam, T.M.; Kunst, L. Extending the Story of Very-Long-Chain Fatty Acid Elongation. Plant Sci. 2013, 210, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, S.P.; Roth, M.R.; Baughman, E.; Li, M.; Tamura, P.; Jeannotte, R.; Welti, R.; Wang, X. Quantitative Profiling of Polar Glycerolipid Species from Organs of Wild-Type Arabidopsis and a PHOSPHOLIPASE Dα1 Knockout Mutant. Phytochemistry 2006, 67, 1907–1924. [Google Scholar] [CrossRef]
- Markham, J.E.; Li, J.; Cahoon, E.B.; Jaworski, J.G. Separation and Identification of Major Plant Sphingolipid Classes from Leaves. J. Biol. Chem. 2006, 281, 22684–22694. [Google Scholar] [CrossRef] [Green Version]
- Markham, J.E.; Lynch, D.V.; Napier, J.A.; Dunn, T.M.; Cahoon, E.B. Plant Sphingolipids: Function Follows Form. Curr. Opin. Plant Biol. 2013, 16, 350–357. [Google Scholar] [CrossRef]
- Buré, C.; Cacas, J.-L.; Wang, F.; Gaudin, K.; Domergue, F.; Mongrand, S.; Schmitter, J.-M. Fast Screening of Highly Glycosylated Plant Sphingolipids by Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3131–3145. [Google Scholar] [CrossRef]
- Carmona-Salazar, L.; Cahoon, R.E.; Gasca-Pineda, J.; González-Solís, A.; Vera-Estrella, R.; Treviño, V.; Cahoon, E.B.; Gavilanes-Ruiz, M. Plasma and Vacuolar Membrane Sphingolipidomes: Composition and Insights on the Role of Main Molecular Species. Plant Physiol. 2021. [Google Scholar] [CrossRef]
- Suh, M.C.; Samuels, A.L.; Jetter, R.; Kunst, L.; Pollard, M.; Ohlrogge, J.; Beisson, F. Cuticular Lipid Composition, Surface Structure, and Gene Expression in Arabidopsis Stem Epidermis. Plant Physiol. 2005, 139, 1649–1665. [Google Scholar] [CrossRef] [Green Version]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic Polyesters in Arabidopsis Surface Tissues—A Typical Suberin and a Particular Cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef] [Green Version]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building Lipid Barriers: Biosynthesis of Cutin And Suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis Cuticular Waxes: Advances in Synthesis, Export and Regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Advances in the Understanding of Cuticular Waxes in Arabidopsis Thaliana and Crop Species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef]
- Kunst, L.; Taylor, D.C.; Underhill, E.W. Fatty Acid Elongation in Developing Seeds of Arabidopsis Thaliana. Plant Physiol. Biochem. 1992, 30, 425–434. [Google Scholar]
- Millar, A.A.; Kunst, L. Very-Long-Chain Fatty Acid Biosynthesis Is Controlled through the Expression and Specificity of the Condensing Enzyme. Plant J. 1997, 12, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.-J.; Go, Y.S.; Park, C.-M. The MYB96 Transcription Factor Regulates Cuticular Wax Biosynthesis under Drought Conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef] [Green Version]
- Hegebarth, D.; Buschhaus, C.; Wu, M.; Bird, D.; Jetter, R. The Composition of Surface Wax on Trichomes of Arabidopsis Thaliana Differs from Wax on Other Epidermal Cells. Plant J. 2016, 88, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Busta, L.; Jetter, R. Structure and Biosynthesis of Branched Wax Compounds on Wild Type and Wax Biosynthesis Mutants of Arabidopsis Thaliana. Plant Cell Physiol. 2017, 58, 1059–1074. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Kosma, D.K.; Tomasi, P.; Dyer, J.M.; Li, R.; Liu, X.; Wang, Z.; Parsons, E.P.; Jenks, M.A.; et al. The Acyl Desaturase CER17 Is Involved in Producing Wax Unsaturated Primary Alcohols and Cutin Monomers. Plant Physiol. 2017, 173, 1109–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busta, L.; Hegebarth, D.; Kroc, E.; Jetter, R. Changes in Cuticular Wax Coverage and Composition on Developing Arabidopsis Leaves Are Influenced by Wax Biosynthesis Gene Expression Levels and Trichome Density. Planta 2017, 245, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Domergue, F.; Vishwanath, S.J.; Joubès, J.; Ono, J.; Lee, J.A.; Bourdon, M.; Alhattab, R.; Lowe, C.; Pascal, S.; Lessire, R.; et al. Three Arabidopsis Fatty Acyl-Coenzyme A Reductases, FAR1, FAR4, and FAR5, Generate Primary Fatty Alcohols Associated with Suberin Deposition. Plant Physiol. 2010, 153, 1539–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlrogge, J.; Thrower, N.; Mhaske, V.; Stymne, S.; Baxter, M.; Yang, W.; Liu, J.; Shaw, K.; Shorrosh, B.; Zhang, M.; et al. PlantFAdb: A Resource for Exploring Hundreds of Plant Fatty Acid Structures Synthesized by Thousands of Plants and Their Phylogenetic Relationships. Plant J. 2018, 96, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Wettstein-Knowles, P. Elongase and Epicuticular Wax Biosynthesis. Physiologie végétale 1982, 20, 797–809. [Google Scholar]
- Schnurr, J.; Shockey, J.; Browse, J. The Acyl-CoA Synthetase Encoded by LACS2 Is Essential for Normal Cuticle Development in Arabidopsis. Plant Cell 2004, 16, 629–642. [Google Scholar] [CrossRef] [Green Version]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing Plant Surfaces: Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Joubès, J.; Raffaele, S.; Bourdenx, B.; Garcia, C.; Laroche-Traineau, J.; Moreau, P.; Domergue, F.; Lessire, R. The VLCFA Elongase Gene Family in Arabidopsis Thaliana: Phylogenetic Analysis, 3D Modelling and Expression Profiling. Plant Mol. Biol. 2008, 67, 547–566. [Google Scholar] [CrossRef]
- Beaudoin, F.; Wu, X.; Li, F.; Haslam, R.P.; Markham, J.E.; Zheng, H.; Napier, J.A.; Kunst, L. Functional Characterization of the Arabidopsis β -Ketoacyl-Coenzyme A Reductase Candidates of the Fatty Acid Elongase. Plant Physiol. 2009, 150, 1174–1191. [Google Scholar] [CrossRef] [Green Version]
- Bach, L.; Michaelson, L.V.; Haslam, R.; Bellec, Y.; Gissot, L.; Marion, J.; Da Costa, M.; Boutin, J.-P.; Miquel, M.; Tellier, F.; et al. The Very-Long-Chain Hydroxy Fatty Acyl-CoA Dehydratase PASTICCINO2 Is Essential and Limiting for Plant Development. Proc. Natl. Acad. Sci. USA 2008, 105, 14727–14731. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Rowland, O.; Kunst, L. Disruptions of the Arabidopsis Enoyl-CoA Reductase Gene Reveal an Essential Role for Very-Long-Chain Fatty Acid Synthesis in Cell Expansion during Plant Morphogenesis. Plant Cell 2005, 17, 1467–1481. [Google Scholar] [CrossRef] [Green Version]
- James, D.W.; Lim, E.; Keller, J.; Plooy, I.; Ralston, E.; Dooner, H.K. Directed Tagging of the Arabidopsis FATTY ACID ELONGATION1 (FAE1) Gene with the Maize Transposon Activator. Plant Cell 1995, 7, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Beaudoin, F.; Gable, K.; Sayanova, O.; Dunn, T.; Napier, J.A. A Saccharomyces Cerevisiae Gene Required for Heterologous Fatty Acid Elongase Activity Encodes a Microsomal β-Keto-Reductase. J. Biol. Chem. 2002, 277, 11481–11488. [Google Scholar] [CrossRef] [Green Version]
- Kohlwein, S.D.; Eder, S.; Oh, C.-S.; Martin, C.E.; Gable, K.; Bacikova, D.; Dunn, T. Tsc13p Is Required for Fatty Acid Elongation and Localizes to a Novel Structure at the Nuclear-Vacuolar Interface In Saccharomyces Cerevisiae. Mol. Cell. Biol. 2001, 21, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Denic, V.; Weissman, J.S. A Molecular Caliper Mechanism for Determining Very Long-Chain Fatty Acid Length. Cell 2007, 130, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.A.; Stenback, K.E.; Flyckt, K.; Hoang, T.; Perera, M.A.D.; Nikolau, B.J. A Single-Cell Platform for Reconstituting and Characterizing Fatty Acid Elongase Component Enzymes. PLoS ONE 2019, 14, e0213620. [Google Scholar] [CrossRef] [Green Version]
- Huai, D.; Xue, X.; Li, Y.; Wang, P.; Li, J.; Yan, L.; Chen, Y.; Wang, X.; Liu, N.; Kang, Y.; et al. Genome-Wide Identification of Peanut KCS Genes Reveals That AhKCS1 and AhKCS28 Are Involved in Regulating VLCFA Contents in Seeds. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, J.; Yang, X.; Jiang, H.; Du, Y.; Liu, X.; Xie, R.; Chai, Y. Genome-Wide Mining and Comparative Analysis of Fatty Acid Elongase Gene Family in Brassica Napus and Its Progenitors. Gene 2020, 747, 144674. [Google Scholar] [CrossRef]
- Dietrich, C.R.; Perera, M.A.D.N.; Yandeau-Nelson, M.D.; Meeley, R.B.; Nikolau, B.J.; Schnable, P.S. Characterization of Two GL8 Paralogs Reveals That the 3-Ketoacyl Reductase Component of Fatty Acid Elongase Is Essential for Maize (Zea mays L.) Development. Plant J. 2005, 42, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Morineau, C.; Gissot, L.; Bellec, Y.; Hematy, K.; Tellier, F.; Renne, C.; Haslam, R.; Beaudoin, F.; Napier, J.; Faure, J.-D. Dual Fatty Acid Elongase Complex Interactions in Arabidopsis. PLoS ONE 2016, 11, e0160631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, T.M.; Kunst, L. Arabidopsis ECERIFERUM2-LIKEs Are Mediators of Condensing Enzyme Function. Plant Cell Physiol. 2020, 61, 2126–2138. [Google Scholar] [CrossRef] [PubMed]
- Haslam, T.M.; Mañas-Fernández, A.; Zhao, L.; Kunst, L. Arabidopsis ECERIFERUM2 Is a Component of the Fatty Acid Elongation Machinery Required for Fatty Acid Extension to Exceptional Lengths. Plant Physiol. 2012, 160, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Haslam, T.M.; Haslam, R.; Thoraval, D.; Pascal, S.; Delude, C.; Domergue, F.; Fernández, A.M.; Beaudoin, F.; Napier, J.A.; Kunst, L.; et al. ECERIFERUM2-LIKE Proteins Have Unique Biochemical and Physiological Functions in Very-Long-Chain Fatty Acid Elongation. Plant Physiol. 2015, 167, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hu, W.; Mishra, N.; Wei, J.; Lu, H.; Hou, Y.; Qiu, X.; Yu, S.; Wang, C.; Zhang, H.; et al. AKR2A Interacts with KCS1 to Improve VLCFAs Contents and Chilling Tolerance of Arabidopsis Thaliana. Plant J. 2020, 103, 1575–1589. [Google Scholar] [CrossRef]
- Trenkamp, S.; Martin, W.; Tietjen, K. Specific and Differential Inhibition of Very-Long-Chain Fatty Acid Elongases from Arabidopsis Thaliana by Different Herbicides. Proc. Natl. Acad. Sci. USA 2004, 101, 11903–11908. [Google Scholar] [CrossRef] [Green Version]
- Tresch, S.; Heilmann, M.; Christiansen, N.; Looser, R.; Grossmann, K. Inhibition of Saturated Very-Long-Chain Fatty Acid Biosynthesis by Mefluidide and Perfluidone, Selective Inhibitors of 3-Ketoacyl-CoA Synthases. Phytochemistry 2012, 76, 162–171. [Google Scholar] [CrossRef]
- Paul, S.; Gable, K.; Beaudoin, F.; Cahoon, E.; Jaworski, J.; Napier, J.A.; Dunn, T.M. Members of the Arabidopsis FAE1-like 3-Ketoacyl-CoA Synthase Gene Family Substitute for the Elop Proteins of Saccharomyces Cerevisiae. J. Biol. Chem. 2006, 281, 9018–9029. [Google Scholar] [CrossRef] [Green Version]
- Blacklock, B.J.; Jaworski, J.G. Substrate Specificity of Arabidopsis 3-Ketoacyl-CoA Synthases. Biochem. Biophys. Res. Commun. 2006, 346, 583–590. [Google Scholar] [CrossRef]
- Hegebarth, D.; Buschhaus, C.; Joubès, J.; Thoraval, D.; Bird, D.; Jetter, R. Arabidopsis Ketoacyl-CoA Synthase 16 (KCS16) Forms C36/C38 Acyl Precursors for Leaf Trichome and Pavement Surface Wax. Plant Cell Environ. 2017, 40, 1761–1776. [Google Scholar] [CrossRef]
- Millar, A.A.; Clemens, S.; Zachgo, S.; Giblin, E.M.; Taylor, D.C.; Kunst, L. CUT1, an Arabidopsis Gene Required for Cuticular Wax Biosynthesis and Pollen Fertility, Encodes a Very-Long-Chain Fatty Acid Condensing Enzyme. Plant Cell 1999, 11, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Hooker, T.S.; Millar, A.A.; Kunst, L. Significance of the Expression of the CER6 Condensing Enzyme for Cuticular Wax Production in Arabidopsis. Plant Physiol. 2002, 129, 1568–1580. [Google Scholar] [CrossRef] [Green Version]
- Franke, R.; Höfer, R.; Briesen, I.; Emsermann, M.; Efremova, N.; Yephremov, A.; Schreiber, L. The DAISY Gene from Arabidopsis Encodes a Fatty Acid Elongase Condensing Enzyme Involved in the Biosynthesis of Aliphatic Suberin in Roots and the Chalaza-Micropyle Region of Seeds. Plant J. 2009, 57, 80–95. [Google Scholar] [CrossRef]
- Lee, S.-B.; Jung, S.-J.; Go, Y.-S.; Kim, H.-U.; Kim, J.-K.; Cho, H.-J.; Park, O.K.; Suh, M.-C. Two Arabidopsis 3-Ketoacyl CoA Synthase Genes, KCS20 and KCS2/DAISY, Are Functionally Redundant in Cuticular Wax and Root Suberin Biosynthesis, but Differentially Controlled by Osmotic Stress. Plant J. 2009, 60, 462–475. [Google Scholar] [CrossRef]
- Todd, J.; Post-Beittenmiller, D.; Jaworski, J.G. KCS1 Encodes a Fatty Acid Elongase 3-Ketoacyl-CoA Synthase Affecting Wax Biosynthesis in Arabidopsis Thaliana. Plant J. 1999, 17, 119–130. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.H.; Lee, S.B.; Go, Y.S.; Kim, H.J.; Cahoon, R.; Markham, J.E.; Cahoon, E.B.; Suh, M.C. Arabidopsis 3-Ketoacyl-Coenzyme A Synthase9 Is Involved in the Synthesis of Tetracosanoic Acids as Precursors of Cuticular Waxes, Suberins, Sphingolipids, and Phospholipids. Plant Physiol. 2013, 162, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, S.B.; Suh, M.C. Arabidopsis 3-Ketoacyl-CoA Synthase 4 Is Essential for Root and Pollen Tube Growth. J. Plant Biol. 2021, 64, 155–165. [Google Scholar] [CrossRef]
- Lv, B.; Wei, K.; Hu, K.; Tian, T.; Zhang, F.; Yu, Z.; Zhang, D.; Su, Y.; Sang, Y.; Zhang, X.; et al. MPK14-Mediated Auxin Signaling Controls Lateral Root Development via ERF13-Regulated Very-Long-Chain Fatty Acid Biosynthesis. Mol. Plant 2021, 14, 285–297. [Google Scholar] [CrossRef]
- Delude, C.; Fouillen, L.; Bhar, P.; Cardinal, M.-J.; Pascal, S.; Santos, P.; Kosma, D.K.; Joubès, J.; Rowland, O.; Domergue, F. Primary Fatty Alcohols Are Major Components of Suberized Root Tissues of Arabidopsis in the Form of Alkyl Hydroxycinnamates. Plant Physiol. 2016, 171, 1934–1950. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, T.; Griffiths, D.W. The Effects of Stress on Plant Cuticular Waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Ingram, G.; Nawrath, C. The Roles of the Cuticle in Plant Development: Organ Adhesions and Beyond. J. Exp. Bot. 2017, 68, 5307–5321. [Google Scholar] [CrossRef]
- Delude, C.; Moussu, S.; Joubès, J.; Ingram, G.; Domergue, F. Plant Surface Lipids and Epidermis Development. In Lipids in Plant and Algae Development; Subcellular Biochemistry; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 287–313. ISBN 978-3-319-25979-6. [Google Scholar]
- Fich, E.A.; Segerson, N.A.; Rose, J.K.C. The Plant Polyester Cutin: Biosynthesis, Structure, and Biological Roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition Differences between Epicuticular and Intracuticular Wax Substructures: How Do Plants Seal Their Epidermal Surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Hegebarth, D.; Jetter, R. Cuticular Waxes of Arabidopsis Thaliana Shoots: Cell-Type-Specific Composition and Biosynthesis. Plants 2017, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Li, X.; Yao, L.; Xu, D.; Li, Y.; Zhang, X.; Li, Z.; Xiao, Q.; Ni, Y.; Guo, Y. Chemical Profiles of Cuticular Waxes on Various Organs of Sorghum Bicolor and Their Antifungal Activities. Plant Physiol. Biochem. 2020, 155, 596–604. [Google Scholar] [CrossRef]
- Fiebig, A.; Mayfield, J.A.; Miley, N.L.; Chau, S.; Fischer, R.L.; Preuss, D. Alterations in CER6, a Gene Identical to CUT1, Differentially Affect Long-Chain Lipid Content on the Surface of Pollen and Stems. Plant Cell 2000, 12, 2001–2008. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guan, Y.; Zhang, D.; Dong, X.; Tian, L.; Qu, L.Q. A β-Ketoacyl-CoA Synthase Is Involved in Rice Leaf Cuticular Wax Synthesis and Requires a CER2-LIKE Protein as a Cofactor. Plant Physiol. 2017, 173, 944–955. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, Z.; Feng, T.; Li, J.; Huang, L.; Yang, B.; Zhao, H.; Jenks, M.A.; Yang, P.; Lü, S. Evolutionarily Conserved Function of the Sacred Lotus (Nelumbo Nucifera Gaertn.) CER2-LIKE Family in Very-Long-Chain Fatty Acid Elongation. Planta 2018, 248, 715–727. [Google Scholar] [CrossRef]
- Alexander, L.E.; Okazaki, Y.; Schelling, M.A.; Davis, A.; Zheng, X.; Rizhsky, L.; Yandeau-Nelson, M.D.; Saito, K.; Nikolau, B.J. Maize Glossy2 and Glossy2-like Genes Have Overlapping and Distinct Functions in Cuticular Lipid Deposition. Plant Physiol. 2020, 183, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Vigil, E.; von Loessl, M.E.; Chen, J.Y.; Li, S.; Haslam, T.M.; Kunst, L.; Mansfield, S.D. Understanding the Role of Populus ECERIFERUM2-Likes in the Biosynthesis of Very-Long-Chain Fatty Acids for Cuticular Waxes. Plant Cell Physiol 2021. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax Biosynthesis in Response to Danger: Its Regulation upon Abiotic and Biotic Stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Jin, S.; Xu, Y.; Li, S.; Zhang, S.; Yuan, Z.; Li, J.; Ni, Y. Overexpression of BnKCS1-1, BnKCS1-2, and BnCER1-2 Promotes Cuticular Wax Production and Increases Drought Tolerance in Brassica Napus. Crop J. 2020, 8, 26–37. [Google Scholar] [CrossRef]
- Lokesh, U.; Venkatesh, B.; Kiranmai, K.; Nareshkumar, A.; Amarnathareddy, V.; Rao, G.L.; Anthony Johnson, A.M.; Pandurangaiah, M.; Sudhakar, C. Overexpression of SS-Ketoacyl Co-A Synthase1 Gene Improves Tolerance of Drought Susceptible Groundnut (Arachis Hypogaea L.) Cultivar K-6 by Increased Leaf Epicuticular Wax Accumulation. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Wu, Q.; Yang, L.; Hu, W.; Liu, D.; Liu, Y. Ectopic Expression of CsKCS6 From Navel Orange Promotes the Production of Very-Long-Chain Fatty Acids (VLCFAs) and Increases the Abiotic Stress Tolerance of Arabidopsis Thaliana. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Weidenbach, D.; Jansen, M.; Bodewein, T.; Nagel, K.A.; Schaffrath, U. Shoot and Root Phenotyping of the Barley Mutant Kcs6 (3-Ketoacyl-CoA Synthase6) Depleted in Epicuticular Waxes under Water Limitation. Plant Signal. Behav. 2015, 10, e1003752. [Google Scholar] [CrossRef]
- Weidenbach, D.; Jansen, M.; Franke, R.B.; Hensel, G.; Weissgerber, W.; Ulferts, S.; Jansen, I.; Schreiber, L.; Korzun, V.; Pontzen, R.; et al. Evolutionary Conserved Function of Barley and Arabidopsis 3-KETOACYL-CoA SYNTHASES in Providing Wax Signals for Germination of Powdery Mildew Fungi. Plant Physiol. 2014, 166, 1621–1633. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Haslam, T.M.; Krüger, A.; Schneider, L.M.; Mishina, K.; Samuels, L.; Yang, H.; Kunst, L.; Schaffrath, U.; Nawrath, C.; et al. The β-Ketoacyl-CoA Synthase HvKCS1, Encoded by Cer-Zh, Plays a Key Role in Synthesis of Barley Leaf Wax and Germination of Barley Powdery Mildew. Plant Cell Physiol. 2018, 59, 811–827. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.-Y.; Lee, Y.N.; Kim, S.-G.; Lee, Y.-H.; Park, W.J.; Park, C.-M. The MYB96 Transcription Factor Mediates Abscisic Acid Signaling during Drought Stress Response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Kim, H.; Kim, R.J.; Suh, M.C. Overexpression of Arabidopsis MYB96 Confers Drought Resistance in Camelina Sativa via Cuticular Wax Accumulation. Plant Cell Rep. 2014, 33, 1535–1546. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Cuticular Wax Biosynthesis Is Up-Regulated by the MYB94 Transcription Factor in Arabidopsis. Plant Cell Physiol. 2015, 56, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Castorina, G.; Domergue, F.; Chiara, M.; Zilio, M.; Persico, M.; Ricciardi, V.; Horner, D.S.; Consonni, G. Drought-Responsive ZmFDL1/MYB94 Regulates Cuticle Biosynthesis and Cuticle-Dependent Leaf Permeability. Plant Physiol. 2020, 184, 266–282. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.U.; Suh, M.C. MYB94 and MYB96 Additively Activate Cuticular Wax Biosynthesis in Arabidopsis. Plant Cell Physiol. 2016, 57, 2300–2311. [Google Scholar] [CrossRef] [Green Version]
- Go, Y.S.; Kim, H.; Kim, H.J.; Suh, M.C. Arabidopsis Cuticular Wax Biosynthesis Is Negatively Regulated by the DEWAX Gene Encoding an AP2/ERF-Type Transcription Factor. Plant Cell 2014, 26, 1666–1680. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.; Dong, Y.; Szymanski, J.; Lashbrooke, J.; Meir, S.; Almekias-Siegl, E.; Zeisler-Diehl, V.V.; Schreiber, L.; Aharoni, A. A Multilevel Study of Melon Fruit Reticulation Provides Insight into Skin Ligno-Suberization Hallmarks. Plant Physiol. 2019, 179, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Legay, S.; Cocco, E.; André, C.M.; Guignard, C.; Hausman, J.-F.; Guerriero, G. Differential Lipid Composition and Gene Expression in the Semi-Russeted “Cox Orange Pippin” Apple Variety. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Graça, J.; Pereira, H. Suberin Structure in Potato Periderm: Glycerol, Long-Chain Monomers, and Glyceryl and Feruloyl Dimers. J. Agric. Food Chem. 2000, 48, 5476–5483. [Google Scholar] [CrossRef]
- Serra, O.; Hohn, C.; Franke, R.; Prat, S.; Molinas, M.; Figueras, M. A Feruloyl Transferase Involved in the Biosynthesis of Suberin and Suberin-Associated Wax Is Required for Maturation and Sealing Properties of Potato Periderm. Plant J. 2010, 62, 277–290. [Google Scholar] [CrossRef]
- Franke, R.B.; Dombrink, I.; Schreiber, L. Suberin Goes Genomics: Use of a Short Living Plant to Investigate a Long Lasting Polymer. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Shang, B.; Xu, C.; Zhang, X.; Cao, H.; Xin, W.; Hu, Y. Very-Long-Chain Fatty Acids Restrict Regeneration Capacity by Confining Pericycle Competence for Callus Formation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 5101–5106. [Google Scholar] [CrossRef] [Green Version]
- Lulai, E.C. Chapter 22—Skin-Set, Wound Healing, and Related Defects. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Mackerron, D.K.L., Taylor, M.A., Ross, H.A., Eds.; Elsevier Science, B.V.: Amsterdam, The Netherlands, 2007; pp. 471–500. ISBN 978-0-444-51018-1. [Google Scholar]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and Suberin Development of Native and Wound Periderm of Potato (Solanum Tuberosum L.) and Its Relation to Peridermal Transpiration. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef]
- Serra, O.; Soler, M.; Hohn, C.; Franke, R.; Schreiber, L.; Prat, S.; Molinas, M.; Figueras, M. Silencing of StKCS6 in Potato Periderm Leads to Reduced Chain Lengths of Suberin and Wax Compounds and Increased Peridermal Transpiration. J. Exp. Bot. 2009, 60, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Nishiuchi, S.; Kulichikhin, K.; Nakazono, M. Does Suberin Accumulation in Plant Roots Contribute to Waterlogging Tolerance? Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Kreszies, T.; Shellakkutti, N.; Osthoff, A.; Yu, P.; Baldauf, J.A.; Zeisler-Diehl, V.V.; Ranathunge, K.; Hochholdinger, F.; Schreiber, L. Osmotic Stress Enhances Suberization of Apoplastic Barriers in Barley Seminal Roots: Analysis of Chemical, Transcriptomic and Physiological Responses. New Phytol. 2019, 221, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Merlin, I.; Pascal, S.; Bert, P.-F.; Domergue, F.; Gambetta, G.A. Drought Activates MYB41 Orthologs and Induces Suberization of Grapevine Fine Roots. Plant Direct 2020, 4, e00278. [Google Scholar] [CrossRef] [PubMed]
- Holbein, J.; Franke, R.B.; Marhavý, P.; Fujita, S.; Górecka, M.; Sobczak, M.; Geldner, N.; Schreiber, L.; Grundler, F.M.W.; Siddique, S. Root Endodermal Barrier System Contributes to Defence against Plant-Parasitic Cyst and Root-Knot Nematodes. Plant J. 2019, 100, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Cacas, J.-L.; Buré, C.; Grosjean, K.; Gerbeau-Pissot, P.; Lherminier, J.; Rombouts, Y.; Maes, E.; Bossard, C.; Gronnier, J.; Furt, F.; et al. Revisiting Plant Plasma Membrane Lipids in Tobacco: A Focus on Sphingolipids. Plant Physiol. 2016, 170, 367–384. [Google Scholar] [CrossRef]
- Pata, M.O.; Hannun, Y.A.; Ng, C.K.-Y. Plant Sphingolipids: Decoding the Enigma of the Sphinx. New Phytol. 2010, 185, 611–630. [Google Scholar] [CrossRef] [Green Version]
- Markham, J.E.; Jaworski, J.G. Rapid Measurement of Sphingolipids from Arabidopsis Thaliana by Reversed-Phase High-Performance Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.C.; Yu, X.; Albrecht, S.; Sicilia, F.; Huichalaf, M.; Ampuero, D.; Michaelson, L.V.; Murphy, A.M.; Matsunaga, T.; Kurz, S.; et al. Abnormal Glycosphingolipid Mannosylation Triggers Salicylic Acid–Mediated Responses in Arabidopsis. Plant Cell 2013, 25, 1881–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tellier, F.; Maia-Grondard, A.; Schmitz-Afonso, I.; Faure, J.-D. Comparative Plant Sphingolipidomic Reveals Specific Lipids in Seeds and Oil. Phytochemistry 2014, 103, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, P.; Feussner, K.; Feussner, I. An Enhanced Plant Lipidomics Method Based on Multiplexed Liquid Chromatography–Mass Spectrometry Reveals Additional Insights into Cold- and Drought-Induced Membrane Remodeling. Plant J. 2015, 84, 621–633. [Google Scholar] [CrossRef]
- Mamode Cassim, A.; Navon, Y.; Gao, Y.; Decossas, M.; Fouillen, L.; Grélard, A.; Nagano, M.; Lambert, O.; Bahammou, D.; Van Delft, P.; et al. Biophysical Analysis of the Plant-Specific GIPC Sphingolipids Reveals Multiple Modes of Membrane Regulation. J. Biol. Chem. 2021, 296, 100602. [Google Scholar] [CrossRef]
- Muralidhar, P.; Radhika, P.; Krishna, N.; Rao, D.V.; Rao, C.B. Sphingolipids from Marine Organisms: A Review. Nat. Prod. Sci. 2003, 9, 117–142. [Google Scholar]
- Řezanka, T.; Kolouchová, I.; Gharwalová, L.; Doležalová, J.; Nedbalová, L.; Sigler, K. Sphingolipidomics of Thermotolerant Yeasts. Lipids 2018, 53, 627–639. [Google Scholar] [CrossRef]
- Markham, J.E.; Molino, D.; Gissot, L.; Bellec, Y.; Hématy, K.; Marion, J.; Belcram, K.; Palauqui, J.-C.; Satiat-JeuneMaître, B.; Faure, J.-D. Sphingolipids Containing Very-Long-Chain Fatty Acids Define a Secretory Pathway for Specific Polar Plasma Membrane Protein Targeting in Arabidopsis. Plant Cell 2011, 23, 2362–2378. [Google Scholar] [CrossRef] [Green Version]
- Msanne, J.; Chen, M.; Luttgeharm, K.D.; Bradley, A.M.; Mays, E.S.; Paper, J.M.; Boyle, D.L.; Cahoon, R.E.; Schrick, K.; Cahoon, E.B. Glucosylceramides Are Critical for Cell-Type Differentiation and Organogenesis, but Not for Cell Viability in Arabidopsis. Plant J. 2015, 84, 188–201. [Google Scholar] [CrossRef] [Green Version]
- Cacas, J.-L.; Buré, C.; Furt, F.; Maalouf, J.-P.; Badoc, A.; Cluzet, S.; Schmitter, J.-M.; Antajan, E.; Mongrand, S. Biochemical Survey of the Polar Head of Plant Glycosylinositolphosphoceramides Unravels Broad Diversity. Phytochemistry 2013, 96, 191–200. [Google Scholar] [CrossRef]
- Martinière, A.; Lavagi, I.; Nageswaran, G.; Rolfe, D.J.; Maneta-Peyret, L.; Luu, D.-T.; Botchway, S.W.; Webb, S.E.D.; Mongrand, S.; Maurel, C.; et al. Cell Wall Constrains Lateral Diffusion of Plant Plasma-Membrane Proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 12805–12810. [Google Scholar] [CrossRef] [Green Version]
- Nagano, M.; Takahara, K.; Fujimoto, M.; Tsutsumi, N.; Uchimiya, H.; Kawai-Yamada, M. Arabidopsis Sphingolipid Fatty Acid 2-Hydroxylases (AtFAH1 and AtFAH2) Are Functionally Differentiated in Fatty Acid 2-Hydroxylation and Stress Responses. Plant Physiol. 2012, 159, 1138–1148. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.A.; Dauk, M.; Ramadan, H.; Yang, H.; Seamons, L.E.; Haslam, R.P.; Beaudoin, F.; Ramirez-Erosa, I.; Forseille, L. Involvement of Arabidopsis ACYL-COENZYME A DESATURASE-LIKE2 (At2g31360) in the Biosynthesis of the Very-Long-Chain Monounsaturated Fatty Acid Components of Membrane Lipids. Plant Physiol. 2013, 161, 81–96. [Google Scholar] [CrossRef] [Green Version]
- König, S.; Feussner, K.; Schwarz, M.; Kaever, A.; Iven, T.; Landesfeind, M.; Ternes, P.; Karlovsky, P.; Lipka, V.; Feussner, I. Arabidopsis Mutants of Sphingolipid Fatty Acid α-Hydroxylases Accumulate Ceramides and Salicylates. New Phytol. 2012, 196, 1086–1097. [Google Scholar] [CrossRef]
- Gronnier, J.; Legrand, A.; Loquet, A.; Habenstein, B.; Germain, V.; Mongrand, S. Mechanisms Governing Subcompartmentalization of Biological Membranes. Curr. Opin. Plant Biol. 2019, 52, 114–123. [Google Scholar] [CrossRef]
- Jaillais, Y.; Ott, T. The Nanoscale Organization of the Plasma Membrane and Its Importance in Signaling: A Proteolipid Perspective. Plant Physiol. 2020, 182, 1682–1696. [Google Scholar] [CrossRef] [Green Version]
- Mamode Cassim, A.; Gouguet, P.; Gronnier, J.; Laurent, N.; Germain, V.; Grison, M.; Boutté, Y.; Gerbeau-Pissot, P.; Simon-Plas, F.; Mongrand, S. Plant Lipids: Key Players of Plasma Membrane Organization and Function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef]
- Borner, G.H.H.; Sherrier, D.J.; Weimar, T.; Michaelson, L.V.; Hawkins, N.D.; MacAskill, A.; Napier, J.A.; Beale, M.H.; Lilley, K.S.; Dupree, P. Analysis of Detergent-Resistant Membranes in Arabidopsis. Evidence for Plasma Membrane Lipid Rafts. Plant Physiol. 2005, 137, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Kaul, K.; Lester, R.L. Characterization of Inositol-Containing Phosphosphingolipids from Tobacco Leaves: Isolation and Identification of Two Novel, Major Lipids: N-Acetylglucosamidoglucuronidoinositol Phosphorylceramide and Glucosamidoglucuronidoinositol Phosphorylceramide. Plant Physiol. 1975, 55, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Toledo, M.S.; Suzuki, E.; Straus, A.H.; Takahashi, H.K. Glycolipids from Paracoccidioides Brasiliensis. Isolation of a Galactofuranose-Containing Glycolipid Reactive with Sera of Patients with Paracoccidioidomycosis. J. Med. Vet. Mycol. 1995, 33, 247–251. [Google Scholar] [CrossRef]
- Tjellström, H.; Hellgren, L.I.; Wieslander, Å.; Sandelius, A.S. Lipid Asymmetry in Plant Plasma Membranes: Phosphate Deficiency-Induced Phospholipid Replacement Is Restricted to the Cytosolic Leaflet. FASEB J. 2010, 24, 1128–1138. [Google Scholar] [CrossRef]
- Gronnier, J.; Germain, V.; Gouguet, P.; Cacas, J.-L.; Mongrand, S. GIPC: Glycosyl Inositol Phospho Ceramides, the Major Sphingolipids on Earth. Plant Signal. Behav. 2016, 11, e1152438. [Google Scholar] [CrossRef] [Green Version]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma Membranes Are Asymmetric in Lipid Unsaturation, Packing and Protein Shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef]
- Raghupathy, R.; Anilkumar, A.A.; Polley, A.; Singh, P.P.; Yadav, M.; Johnson, C.; Suryawanshi, S.; Saikam, V.; Sawant, S.D.; Panda, A.; et al. Transbilayer Lipid Interactions Mediate Nanoclustering of Lipid-Anchored Proteins. Cell 2015, 161, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Mora-Ranjeva, M.P.; Charveron, M.; Fabre, B.; Milon, A.; Muller, I. Incorporation of Phytosterols in Human Keratinocytes: Consequences on UVA-Induced Lipid Peroxidation and Calcium Ionophore-Induced Prostaglandin Release. Chem. Phys. Lipids 2006, 141, 216–224. [Google Scholar] [CrossRef]
- Grosjean, K.; Mongrand, S.; Beney, L.; Simon-Plas, F.; Gerbeau-Pissot, P. Differential Effect of Plant Lipids on Membrane Organization: SPECIFICITIES OF PHYTOSPHINGOLIPIDS AND PHYTOSTEROLS. J. Biol. Chem. 2015, 290, 5810–5825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, J.G.; Mathieu, D.; Loudet, C.; Buchoux, S.; Dufourc, E.J. Plant Sterols in “Rafts”: A Better Way to Regulate Membrane Thermal Shocks. FASEB J. 2007, 21, 1714–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resemann, H.C.; Herrfurth, C.; Feussner, K.; Hornung, E.; Ostendorf, A.K.; Gömann, J.; Mittag, J.; van Gessel, N.; de Vries, J.; Ludwig-Müller, J.; et al. Convergence of Sphingolipid Desaturation across over 500 Million Years of Plant Evolution. Nat. Plants 2021, 7, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, L.; Fu, X.; Mei, X.; Cheng, S.; Liao, Y.; Deng, R.; Xu, X.; Jiang, Y.; Duan, X.; et al. The Sphingolipid Biosynthetic Enzyme Sphingolipid Delta8 Desaturase Is Important for Chilling Resistance of Tomato. Sci. Rep. 2016, 6, 38742. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Markham, J.E.; Cahoon, E.B. Sphingolipid Δ8 Unsaturation Is Important for Glucosylceramide Biosynthesis and Low-Temperature Performance in Arabidopsis. Plant J. 2012, 69, 769–781. [Google Scholar] [CrossRef]
- Murakami, Y.; Tsuyama, M.; Kobayashi, Y.; Kodama, H.; Iba, K. Trienoic Fatty Acids and Plant Tolerance of High Temperature. Science 2000, 287, 476–479. [Google Scholar] [CrossRef]
- Routaboul, J.-M.; Skidmore, C.; Wallis, J.G.; Browse, J. Arabidopsis Mutants Reveal That Short- and Long-Term Thermotolerance Have Different Requirements for Trienoic Fatty Acids. J. Exp. Bot. 2012, 63, 1435–1443. [Google Scholar] [CrossRef] [Green Version]
- Tovuu, A.; Zulfugarov, I.S.; Wu, G.; Kang, I.S.; Kim, C.; Moon, B.Y.; An, G.; Lee, C.-H. Rice Mutants Deficient in ω-3 Fatty Acid Desaturase (FAD8) Fail to Acclimate to Cold Temperatures. Plant Physiol. Biochem. 2016, 109, 525–535. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant Cell-Surface GIPC Sphingolipids Sense Salt to Trigger Ca 2+ Influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. How Plants Perceive Salt. Nature 2019, 572, 318–320. [Google Scholar] [CrossRef] [Green Version]
- Michaelson, L.V.; Napier, J.A.; Molino, D.; Faure, J.-D. Plant Sphingolipids: Their Importance in Cellular Organization and Adaption. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1329–1335. [Google Scholar] [CrossRef]
- Xie, L.-J.; Chen, Q.-F.; Chen, M.-X.; Yu, L.-J.; Huang, L.; Chen, L.; Wang, F.-Z.; Xia, F.-N.; Zhu, T.-R.; Wu, J.-X.; et al. Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in Arabidopsis. PLoS Genet. 2015, 11, e1005143. [Google Scholar] [CrossRef]
- Xie, L.-J.; Yu, L.-J.; Chen, Q.-F.; Wang, F.-Z.; Huang, L.; Xia, F.-N.; Zhu, T.-R.; Wu, J.-X.; Yin, J.; Liao, B.; et al. Arabidopsis Acyl-CoA-Binding Protein ACBP3 Participates in Plant Response to Hypoxia by Modulating Very-Long-Chain Fatty Acid Metabolism. Plant J. 2015, 81, 53–67. [Google Scholar] [CrossRef]
- Michaelson, L.V. New Insights into Cell Death Induced by Long Chain Bases in Arabidopsis. New Phytol. 2011, 191, 909–911. [Google Scholar] [CrossRef] [Green Version]
- Donahue, J.L.; Alford, S.R.; Torabinejad, J.; Kerwin, R.E.; Nourbakhsh, A.; Ray, W.K.; Hernick, M.; Huang, X.; Lyons, B.M.; Hein, P.P.; et al. The Arabidopsis Thaliana Myo-Inositol 1-Phosphate Synthase1 Gene Is Required for Myo-Inositol Synthesis and Suppression of Cell Death. Plant Cell 2010, 22, 888–903. [Google Scholar] [CrossRef] [Green Version]
- Saucedo-García, M.; Guevara-García, A.; González-Solís, A.; Cruz-García, F.; Vázquez-Santana, S.; Markham, J.E.; Lozano-Rosas, M.G.; Dietrich, C.R.; Ramos-Vega, M.; Cahoon, E.B.; et al. MPK6, Sphinganine and the LCB2a Gene from Serine Palmitoyltransferase Are Required in the Signaling Pathway That Mediates Cell Death Induced by Long Chain Bases in Arabidopsis. New Phytol. 2011, 191, 943–957. [Google Scholar] [CrossRef] [Green Version]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and Plant Defense/Disease: The “Death” Connection and Beyond. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Ali, U.; Li, H.; Wang, X.; Guo, L. Emerging Roles of Sphingolipid Signaling in Plant Response to Biotic and Abiotic Stresses. Mol. Plant 2018, 11, 1328–1343. [Google Scholar] [CrossRef] [Green Version]
- Vailleau, F.; Daniel, X.; Tronchet, M.; Montillet, J.-L.; Triantaphylidès, C.; Roby, D. A R2R3-MYB Gene, AtMYB30, Acts as a Positive Regulator of the Hypersensitive Cell Death Program in Plants in Response to Pathogen Attack. Proc. Natl. Acad. Sci. USA 2002, 99, 10179–10184. [Google Scholar] [CrossRef] [Green Version]
- Raffaele, S.; Vailleau, F.; Léger, A.; Joubès, J.; Miersch, O.; Huard, C.; Blée, E.; Mongrand, S.; Domergue, F.; Roby, D. A MYB Transcription Factor Regulates Very-Long-Chain Fatty Acid Biosynthesis for Activation of the Hypersensitive Cell Death Response in Arabidopsis. Plant Cell 2008, 20, 752–767. [Google Scholar] [CrossRef] [Green Version]
- De Bigault Du Granrut, A.; Cacas, J.-L. How Very-Long-Chain Fatty Acids Could Signal Stressful Conditions in Plants? Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Peraro, M.D.; van der Goot, F.G. Pore-Forming Toxins: Ancient, but Never Really out of Fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Raaymakers, T.M.; Van den Ackerveken, G. Extracellular Recognition of Oomycetes during Biotrophic Infection of Plants. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Lenarčič, T.; Albert, I.; Böhm, H.; Hodnik, V.; Pirc, K.; Zavec, A.B.; Podobnik, M.; Pahovnik, D.; Žagar, E.; Pruitt, R.; et al. Eudicot Plant-Specific Sphingolipids Determine Host Selectivity of Microbial NLP Cytolysins. Science 2017, 358, 1431–1434. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, G.; Jia, Y.; Yu, X.; Zhang, X.; Yu, B.; Wang, D.; Zheng, Y.; Tian, X.; Li, W. Acyl Chain Length of Phosphatidylserine Is Correlated with Plant Lifespan. PLoS ONE 2014, 9, e103227. [Google Scholar] [CrossRef] [Green Version]
- Rani, M.H.; Liu, Q.; Yu, N.; Zhang, Y.; Wang, B.; Cao, Y.; Zhang, Y.; Islam, M.A.; Zegeye, W.A.; Cao, L.; et al. ES5 Is Involved in the Regulation of Phosphatidylserine Synthesis and Impacts on Early Senescence in Rice (Oryza sativa L.). Plant Mol. Biol. 2020, 102, 501–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platre, M.P.; Bayle, V.; Armengot, L.; Bareille, J.; Marquès-Bueno, M.d.M.; Creff, A.; Maneta-Peyret, L.; Fiche, J.-B.; Nollmann, M.; Miège, C.; et al. Developmental Control of Plant Rho GTPase Nano-Organization by the Lipid Phosphatidylserine. Science 2019, 364, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skotland, T.; Sandvig, K. The Role of PS 18:0/18:1 in Membrane Function. Nat. Commun. 2019, 10, 2752. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Holroyd, G.H.; van der Lee, F.M.; Bahrami, A.R.; Sijmons, P.C.; Woodward, F.I.; Schuch, W.; Hetherington, A.M. The HIC Signalling Pathway Links CO 2 Perception to Stomatal Development. Nature 2000, 408, 713–716. [Google Scholar] [CrossRef]
- Bird, S.M.; Gray, J.E. Signals from the Cuticle Affect Epidermal Cell Differentiation. New Phytol. 2003, 157, 9–23. [Google Scholar] [CrossRef]
- Yephremov, A.; Wisman, E.; Huijser, P.; Huijser, C.; Wellesen, K.; Saedler, H. Characterization of the FIDDLEHEAD Gene of Arabidopsis Reveals a Link between Adhesion Response and Cell Differentiation in the Epidermis. Plant Cell 1999, 11, 2187–2201. [Google Scholar] [CrossRef] [Green Version]
- Wolters-Arts, M.; Lush, W.M.; Mariani, C. Lipids Are Required for Directional Pollen-Tube Growth. Nature 1998, 392, 818–821. [Google Scholar] [CrossRef]
- Nagata, K.; Ishikawa, T.; Kawai-Yamada, M.; Takahashi, T.; Abe, M. Ceramides Mediate Positional Signals in Arabidopsis Thaliana Protoderm Differentiation. Development 2021, 148. [Google Scholar] [CrossRef]
- Wattelet-Boyer, V.; Brocard, L.; Jonsson, K.; Esnay, N.; Joubès, J.; Domergue, F.; Mongrand, S.; Raikhel, N.; Bhalerao, R.P.; Moreau, P.; et al. Enrichment of Hydroxylated C24- and C26-Acyl-Chain Sphingolipids Mediates PIN2 Apical Sorting at Trans -Golgi Network Subdomains. Nat. Commun. 2016, 7, 12788. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells 2021, 10, 1284. https://doi.org/10.3390/cells10061284
Batsale M, Bahammou D, Fouillen L, Mongrand S, Joubès J, Domergue F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells. 2021; 10(6):1284. https://doi.org/10.3390/cells10061284
Chicago/Turabian StyleBatsale, Marguerite, Delphine Bahammou, Laetitia Fouillen, Sébastien Mongrand, Jérôme Joubès, and Frédéric Domergue. 2021. "Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses" Cells 10, no. 6: 1284. https://doi.org/10.3390/cells10061284