BKCa Channel Inhibition by Peripheral Nerve Injury Is Restored by the Xanthine Derivative KMUP-1 in Dorsal Root Ganglia
Abstract
:1. Introduction
2. Materials and Methods
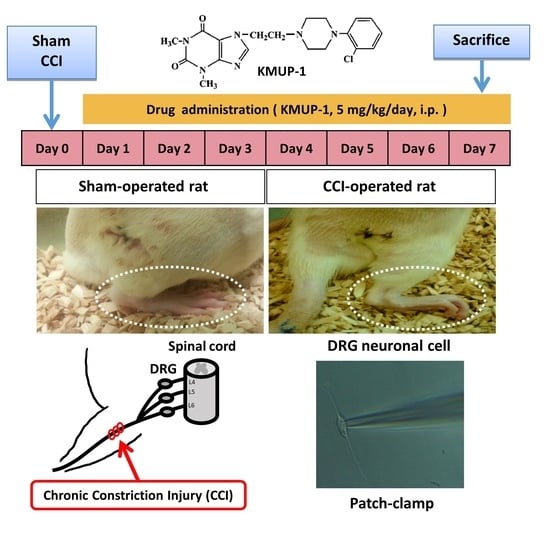
2.1. CCI Animal Model
2.2. Isolation of DRG Cells and Cell Culture
2.3. Perforated and Inside-Out Patch-Clamp Electrophysiology
2.4. Immunofluorescent Staining
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Chemicals
2.7. Data Analysis and Statistics
3. Results
3.1. DRG Neuronal Cells Express Functional BKCa Channels
3.2. KMUP-1 Prevents CCI-Induced Inhibition of BKCa-Channel Activity and Current
3.3. KMUP-1 Restores CCI-Inhibited BKCa Channel Proteins and mRNA Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, V.R.; Sauzet, O.; Williams, J.E.; Ayis, S.; Farquhar-Smith, P.; Ross, J.R.; Branford, R.A.; Peacock, J.L. Adverse event reporting in randomised controlled trials of neuropathic pain: Considerations for future practice. Pain 2013, 154, 213–220. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilron, I.; Jensen, T.S.; Dickenson, A.H. Combination pharmacotherapy for management of chronic pain: From bench to bedside. Lancet Neurol. 2013, 12, 1084–1095. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002, 82, 981–1011. [Google Scholar] [CrossRef] [PubMed]
- Petho, G.; Reeh, P.W. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol. Rev. 2012, 92, 1699–1775. [Google Scholar] [CrossRef] [PubMed]
- Jancalek, R.; Dubovy, P.; Svizenska, I.; Klusakova, I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J. Neuroinflamm. 2010, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Walters, E.T. Injury-related behavior and neuronal plasticity: An evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int. Rev. Neurobiol. 1994, 36, 325–427. [Google Scholar]
- Zimmermann, M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 23–37. [Google Scholar] [CrossRef]
- Ji, R.R.; Kohno, T.; Moore, K.A.; Woolf, C.J. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003, 26, 696–705. [Google Scholar] [CrossRef]
- Sah, P. Ca(2+)-activated K+ currents in neurones: Types, physiological roles and modulation. Trends Neurosci. 1996, 19, 150–154. [Google Scholar] [CrossRef]
- Chen, S.R.; Cai, Y.Q.; Pan, H.L. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J. Neurochem. 2009, 110, 352–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, B.D.; Ahrendt, E.; Braun, A.P.; Braun, J.E. The large conductance, calcium-activated K+ (BK) channel is regulated by cysteine string protein. Sci. Rep. 2013, 3, 2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.H.; Chen, S.R.; Li, L.; Pan, H.L. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J. Neurochem. 2012, 121, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.N.; Lin, R.J.; Lin, C.Y.; Shen, K.P.; Chiang, L.C.; Chen, I.J. A xanthine-based KMUP-1 with cyclic GMP enhancing and K(+) channels opening activities in rat aortic smooth muscle. Br. J. Pharmacol. 2001, 134, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.J.; Wu, B.N.; Lo, Y.C.; Shen, K.P.; Lin, Y.T.; Huang, C.H.; Chen, I.J. KMUP-1 relaxes rabbit corpus cavernosum smooth muscle in vitro and in vivo: Involvement of cyclic GMP and K(+) channels. Br. J. Pharmacol. 2002, 135, 1159–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.N.; Lin, R.J.; Lo, Y.C.; Shen, K.P.; Wang, C.C.; Lin, Y.T.; Chen, I.J. KMUP-1, a xanthine derivative, induces relaxation of guinea-pig isolated trachea: The role of the epithelium, cyclic nucleotides and K+ channels. Br. J. Pharmacol. 2004, 142, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.L.; Hsu, J.H.; Wu, P.J.; Liou, S.F.; Liu, C.P.; Chen, I.J.; Wu, B.N.; Dai, Z.K.; Wu, J.R. KMUP-1 attenuates isoprenaline-induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br. J. Pharmacol. 2010, 159, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.K.; Cheng, Y.J.; Chung, H.H.; Wu, J.R.; Chen, I.J.; Wu, B.N. KMUP-1 ameliorates monocrotaline-induced pulmonary arterial hypertension through the modulation of Ca2+ sensitization and K+-channel. Life Sci. 2010, 86, 747–755. [Google Scholar] [CrossRef]
- Chung, H.H.; Dai, Z.K.; Wu, B.N.; Yeh, J.L.; Chai, C.Y.; Chu, K.S.; Liu, C.P.; Chen, I.J. The xanthine derivative KMUP-1 inhibits models of pulmonary artery hypertension via increased NO and cGMP-dependent inhibition of RhoA/Rho kinase. Br. J. Pharmacol. 2010, 160, 971–986. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.N.; Tu, H.F.; Welsh, D.G.; Chen, I.J. KMUP-1 activates BKCa channels in basilar artery myocytes via cyclic nucleotide-dependent protein kinases. Br. J. Pharmacol. 2005, 146, 862–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Jiang, M.C.; Chu, L.W.; Hsieh, S.L.; Chen, I.J.; Wu, B.N. KMUP-1 inhibits L-type Ca(2)(+) channels involved the protein kinase C in rat basilar artery myocytes. Kaohsiung J. Med. Sci. 2011, 27, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.C.; Tseng, Y.T.; Liu, C.M.; Wu, B.N.; Wu, S.N. Actions of KMUP-1, a xanthine and piperazine derivative, on voltage-gated Na(+) and Ca(2+)-activated K(+) currents in GH3 pituitary tumour cells. Br. J. Pharmacol. 2015, 172, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Cheng, K.I.; Tsai, Y.L.; Hong, Y.R.; Howng, S.L.; Kwan, A.L.; Chen, I.J.; Wu, B.N. Potassium-channel openers KMUP-1 and pinacidil prevent subarachnoid hemorrhage-induced vasospasm by restoring the BKCa-channel activity. Shock 2012, 38, 203–212. [Google Scholar] [CrossRef]
- Ocana, M.; Cendan, C.M.; Cobos, E.J.; Entrena, J.M.; Baeyens, J.M. Potassium channels and pain: Present realities and future opportunities. Eur. J. Pharmacol. 2004, 500, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.K.; Lin, T.C.; Liou, J.C.; Cheng, K.I.; Chen, J.Y.; Chu, L.W.; Chen, I.J.; Wu, B.N. Xanthine derivative KMUP-1 reduces inflammation and hyperalgesia in a bilateral chronic constriction injury model by suppressing MAPK and NFκB activation. Mol. Pharm. 2014, 11, 1621–1631. [Google Scholar] [CrossRef]
- Datta, S.; Chatterjee, K.; Kline, R.H.t.; Wiley, R.G. Behavioral and anatomical characterization of the bilateral sciatic nerve chronic constriction (bCCI) injury: Correlation of anatomic changes and responses to cold stimuli. Mol. Pain 2010, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Vierck, C.J.; Acosta-Rua, A.J.; Johnson, R.D. Bilateral chronic constriction of the sciatic nerve: A model of long-term cold hyperalgesia. J. Pain 2005, 6, 507–517. [Google Scholar] [CrossRef]
- Chu, L.W.; Chen, J.Y.; Wu, P.C.; Wu, B.N. Atorvastatin prevents neuroinflammation in chronic constriction injury rats through nuclear NFκB downregulation in the dorsal root ganglion and spinal cord. ACS Chem. Neurosci. 2015, 6, 889–898. [Google Scholar] [CrossRef]
- Cheng, C.M.; Lin, Y.W.; Bellin, R.M.; Steward, R.L., Jr.; Cheng, Y.R.; LeDuc, P.R.; Chen, C.C. Probing localized neural mechanotransduction through surface-modified elastomeric matrices and electrophysiology. Nat. Protoc. 2010, 5, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Walters, E.T.; Song, X.J. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J. Neurophysiol. 2007, 97, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.H.; Byun, H.S.; Chen, S.R.; Cai, Y.Q.; Pan, H.L. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: Role of brain-derived neurotrophic factor. J. Neurochem. 2010, 114, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, J.H.; Leffler, C.W.; Cheranov, S.Y.; Tcheranova, D.; E, S.; Cheng, X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ. Res. 2002, 91, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Rivero-Melián, C.; Robertson, B.; Grant, G. Transganglionic transport and binding of the isolectin B4 from Griffonia simplicifolia I in rat primary sensory neurons. Neuroscience 1994, 62, 539–551. [Google Scholar] [CrossRef]
- Smith, P.A. K(+) Channels in Primary Afferents and Their Role in Nerve Injury-Induced Pain. Front Cell Neurosci. 2020, 14, 566418. [Google Scholar] [CrossRef]
- Zhang, F.X.; Gadotti, V.M.; Souza, I.A.; Chen, L.; Zamponi, G.W. BK Potassium Channels Suppress Cavα2δ Subunit Function to Reduce Inflammatory and Neuropathic Pain. Cell Rep. 2018, 22, 1956–1964. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Li, L.; Xie, F.; Du, J.; Zuo, Y.; Frost, J.A.; Carlton, S.M.; Walters, E.T.; Yang, Q. Activation of KCNQ Channels Suppresses Spontaneous Activity in Dorsal Root Ganglion Neurons and Reduces Chronic Pain after Spinal Cord Injury. J. Neurotrauma 2017, 34, 1260–1270. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.-I.; Yang, K.-T.; Kung, C.-L.; Cheng, Y.-C.; Yeh, J.-L.; Dai, Z.-K.; Wu, B.-N. BKCa Channel Inhibition by Peripheral Nerve Injury Is Restored by the Xanthine Derivative KMUP-1 in Dorsal Root Ganglia. Cells 2021, 10, 949. https://doi.org/10.3390/cells10040949
Cheng K-I, Yang K-T, Kung C-L, Cheng Y-C, Yeh J-L, Dai Z-K, Wu B-N. BKCa Channel Inhibition by Peripheral Nerve Injury Is Restored by the Xanthine Derivative KMUP-1 in Dorsal Root Ganglia. Cells. 2021; 10(4):949. https://doi.org/10.3390/cells10040949
Chicago/Turabian StyleCheng, Kuang-I, Kan-Ting Yang, Chien-Lun Kung, Yu-Chi Cheng, Jwu-Lai Yeh, Zen-Kong Dai, and Bin-Nan Wu. 2021. "BKCa Channel Inhibition by Peripheral Nerve Injury Is Restored by the Xanthine Derivative KMUP-1 in Dorsal Root Ganglia" Cells 10, no. 4: 949. https://doi.org/10.3390/cells10040949