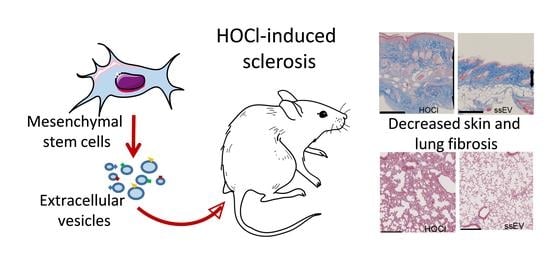
Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mesenchymal Stromal Cell Expansion
2.2. Production and Isolation of EVs
2.3. Animal Model and Histopathological Analysis
2.4. RNA Extraction and RT-qPCR
2.4.1. Measure of Advanced Oxidation Protein Products
2.4.2. Measure of Anti-Oxidant Capacity
2.5. ELISA
2.6. Analysis of miRNA Profiles
2.7. Gene Ontology Pathway Analysis
2.8. Statistical Analysis
3. Results
3.1. A High Dose of MSC-EVs Was Not Beneficial to SSc Mice
3.2. Low Dose IFNγ Pre-Activation Improved the Anti-Fibrotic Effect of MSC-EVs in Lungs
3.3. High Dose IFNγ Pre-Activation Improved Remodeling and Anti-Inflammatory Effect of MSC-EVs in Lungs
3.4. IFNγ Pre-Activation Up-Regulated Anti-Inflammatory Factors in MSCs and MSC-EVs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Tyndall, A. Hematopoietic Stem Cell Transplantation for Systemic Sclerosis: Review of Current Status. BioDrugs 2019, 33, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Rozier, P.; Maria, A.; Goulabchand, R.; Jorgensen, C.; Guilpain, P.; Noël, D. Mesenchymal Stem Cells in Systemic Sclerosis: Allogenic or Autologous Approaches for Therapeutic Use? Front. Immunol. 2018, 9, 2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Maria, A.T.J.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.-C.; Petit, B.; Roger, P.; Batteux, F.; Le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef]
- Maria, A.T.; Toupet, K.; Maumus, M.; Fonteneau, G.; Le Quellec, A.; Jorgensen, C.; Guilpain, P.; Noël, D. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J. Autoimmun. 2016, 70, 31–39. [Google Scholar] [CrossRef]
- Rozier, P.; Maumus, M.; Maria, A.T.J.; Toupet, K.; Lai-Kee-Him, J.; Jorgensen, C.; Guilpain, P.; Noël, D. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J. Autoimmun. 2021, 121, 102660. [Google Scholar] [CrossRef]
- Sivanathan, K.N.; Gronthos, S.; Rojas-Canales, D.; Thierry, B.; Coates, P.T. Interferon-Gamma Modification of Mesenchymal Stem Cells: Implications of Autologous and Allogeneic Mesenchymal Stem Cell Therapy in Allotransplantation. Stem Cell Rev. Rep. 2014, 10, 351–375. [Google Scholar] [CrossRef]
- Szabó, E.; Fajka-Boja, R.; Kriston-Pál, E.; Hornung, A.; Makra, I.; Kudlik, G.; Uher, F.; Katona, R.L.; Monostori, E.; Czibula, A. Licensing by Inflammatory Cytokines Abolishes Heterogeneity of Immunosuppressive Function of Mesenchymal Stem Cell Population. Stem Cells Dev. 2015, 24, 2171–2180. [Google Scholar] [CrossRef] [Green Version]
- Wobma, H.M.; Kanai, M.; Ma, S.P.; Shih, Y.; Li, H.W.; Duran-Struuck, R.; Winchester, R.; Goeta, S.; Brown, L.M.; Vunjak-Novakovic, G. Dual IFN-γ/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms. J. Immunol. Regen. Med. 2018, 1, 45–56. [Google Scholar] [CrossRef]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA–gene interactions. Nucleic Acids Res. 2017, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gregorius, J.; Wang, C.; Stambouli, O.; Hussner, T.; Qi, Y.; Tertel, T.; Börger, V.; Yusuf, A.M.; Hagemann, N.; Yin, D.; et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res. Cardiol. 2021, 116, 1–19. [Google Scholar] [CrossRef]
- Paudyal, A.; Ghinea, F.S.; Driga, M.P.; Fang, W.-H.; Alessandri, G.; Combes, L.; Degens, H.; Slevin, M.; Hermann, D.M.; Popa-Wagner, A. p5 Peptide-Loaded Human Adipose-Derived Mesenchymal Stem Cells Promote Neurological Recovery After Focal Cerebral Ischemia in a Rat Model. Transl. Stroke Res. 2020, 12, 125–135. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-γ–primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli–induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P.; et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2, 21927. [Google Scholar] [CrossRef]
- Ruppert, K.A.; Nguyen, T.T.; Prabhakara, K.S.; Furman, N.E.T.; Srivastava, A.; Harting, M.; Cox, C.S., Jr.; Olson, S.D. Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Modify Microglial Response and Improve Clinical Outcomes in Experimental Spinal Cord Injury. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Serejo, T.R.T.; Silva-Carvalho, A.; Braga, L.D.D.C.F.; Neves, F.D.A.R.; Pereira, R.W.; De Carvalho, J.L.; Saldanha-Araujo, F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells 2019, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Peltzer, J.; Lund, K.; Goriot, M.-E.; Grosbot, M.; Lataillade, J.-J.; Mauduit, P.; Banzet, S. Interferon-γ and Hypoxia Priming Have Limited Effect on the miRNA Landscape of Human Mesenchymal Stromal Cells-Derived Extracellular Vesicles. Front. Cell Dev. Biol. 2020, 8, 1343. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Lindoso, R.S.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S.; et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng. Part A 2017, 23, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Toupet, K.; Maumus, M.; Crawford, P.L.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef] [PubMed]






| Gene Name | Sequence Forward | Sequence Reverse |
|---|---|---|
| Acta2(αSma) | AAGGCCAACCGGGAGAAAAT | AGCCAAGTCCAGACGCATGA |
| Col1a1 | TGTTCAGCTTTGTGGACCTC | TCAAGCATACCTCGGGTTTC |
| Col3a1 | CGGTGAACGGGGCGAAGCTGGTT | GACCCCTTTCTCCTGCGGCTCCT |
| Cox2 | GCATTCTTTGCCCAGCACTT | AGACCAGGCACCAGACCAAAGA |
| Gapdh | GGTGCTGAGTATGTCGTGGA | GTGGTTCACACCCATCACAA |
| Hgf | TGCCCTATTTCCCGTTGTGA | CGCTTCTCCTCGCCTCTCTC |
| Hmox1 | GCAGAGCCGTCTCGAGCATA | GCATTCTCGGCTTGGATGTG |
| Il1ra | AGGCCCCACCACCAGCTTTGA | GGGGCTCTTCCGGTGTGTTGGT |
| Il1β | TTTGACAGTGATGAGAATGACCTGTTC | TCATCAGGACAGCCCAGGTCAAAG |
| Il6 | TGGGACTGATGCTGGTGACA | TTCCACGATTTCCCAGAGAACA |
| iNos | CCTTGTTCAGCTACGCCTTC | GCTTGTCACCACCAGCAGTA |
| Mmp1 | TTCAAAGGCAGCAAAGTATGGGCT | CCAGTCTCTTCTTCACAAACAGCAGCA |
| Mmp9 | TCCAGTTTGGTGTCGCGGAGCACG | CAGGGGGAAAGGCGTGTGCCAGA |
| Nfe2l2 | CGCCAGCTACTCCCAGGTTG | ACTTTCAGCGTGGCTGGGGA |
| Sod2 | TCAGGACCCATTGCAAGGAA | TGTGGCCGTGAGTGACGTTT |
| Tbp | GGGAGAATCATGGACCAGAA | CCGTAAGGCATCATTGGACT |
| Tgfβ1 | TGCGCTTGCAGAGATTAAAA | CTGCCGTACAACTCCAGTGA |
| TgfβR2 | CGACCCCAAGCTCACCTACC | CAACAACAGGTCGGGACTGC |
| Timp1 | CTCCGCCCTTCGCATGGACATT | GGGGGCCATCATGGTATCTGCTCT |
| Tnfα | AGCCCACGTCGTAGCAAACCA | TGTCTTTGAGATCCATGCCGTTGGC |
| MSC | ssEV | lsEV | ||||
|---|---|---|---|---|---|---|
| −IFNγ | +IFNγ | −IFNγ | +IFNγ | −IFNγ | + IFNγ | |
| let-7a-5p | 39,871 | 33,617 | 10,128 | 5963 | 21,047 | 21,213 |
| let-7b-5p | 47,447 | 35,255 | 8648 | 4556 | 21,621 | 21,476 |
| let-7c-3p | 403 | 0 | 0 | 0 | 0 | 0 |
| let-7c-5p | 57,948 | 42,747 | 11,281 | 6154 | 26,131 | 26,347 |
| let-7d-3p | 1097 | 879 | 1585 | 1000 | 1544 | 1455 |
| let-7d-5p | 17,079 | 16,250 | 5002 | 3356 | 9475 | 8869 |
| let-7e-3p | 253 | 0 | 0 | 0 | 0 | 0 |
| let-7e-5p | 7938 | 6261 | 1621 | 1023 | 4224 | 4091 |
| let-7f-1-3p | 245 | 114 | 0 | 0 | 0 | 0 |
| let-7f-5p | 21,504 | 21,024 | 8136 | 4975 | 15,443 | 15,217 |
| let-7g-5p | 6287 | 7170 | 3143 | 2408 | 4563 | 4024 |
| let-7i-5p | 33,329 | 30,098 | 8184 | 4723 | 16,303 | 13,232 |
| miR-100-5p | 7165 | 7546 | 2619 | 1675 | 5070 | 4458 |
| miR-101-3p | 513 | 1572 | 1757 | 2085 | 1188 | 1221 |
| miR-103a-2-5p | 367 | 168 | 0 | 0 | 0 | 0 |
| miR-103a-3p | 2655 | 2334 | 1695 | 1429 | 1978 | 1613 |
| miR-106b-3p | 521 | 275 | 0 | 631 | 0 | 0 |
| miR-106b-5p | 1881 | 2134 | 3213 | 3287 | 2228 | 2239 |
| miR-107 | 1667 | 1684 | 1127 | 1070 | 1170 | 0 |
| miR-1247-5p | 2752 | 1116 | 0 | 598 | 1544 | 1207 |
| miR-1249 | 1213 | 626 | 4544 | 4571 | 3003 | 2429 |
| miR-125a-3p | 745 | 328 | 0 | 0 | 0 | 0 |
| miR-125a-5p | 13,783 | 15,755 | 5304 | 3068 | 11,867 | 10,808 |
| miR-125b-1-3p | 3316 | 944 | 0 | 0 | 0 | 0 |
| miR-125b-5p | 55,996 | 50,774 | 15,833 | 8527 | 31,962 | 31,144 |
| miR-126-3p | 0 | 0 | 4183 | 4440 | 1692 | 1555 |
| miR-126-5p | 0 | 0 | 2105 | 2089 | 1070 | 0 |
| miR-128-3p | 454 | 424 | 0 | 668 | 0 | 0 |
| miR-130a-3p | 7587 | 9334 | 3461 | 1860 | 5813 | 5799 |
| miR-130b-3p | 1339 | 2413 | 1193 | 821 | 2200 | 2280 |
| miR-140-5p | 1309 | 1419 | 0 | 0 | 1333 | 0 |
| miR-142-5p | 0 | 0 | 5341 | 5417 | 2163 | 1799 |
| miR-143-3p | 27,877 | 31,702 | 14,591 | 8415 | 25,698 | 21,680 |
| miR-144-3p | 0 | 0 | 12,449 | 18,149 | 3434 | 2808 |
| miR-145-5p | 37,004 | 33,556 | 16,311 | 8731 | 36,226 | 35,290 |
| miR-146b-5p | 4779 | 7806 | 1639 | 1519 | 3429 | 4867 |
| miR-148a-3p | 2180 | 2622 | 1247 | 903 | 1602 | 2148 |
| miR-148b-3p | 1009 | 974 | 0 | 728 | 0 | 0 |
| miR-149-5p | 1796 | 720 | 0 | 0 | 1292 | 0 |
| miR-150-5p | 0 | 0 | 3595 | 3486 | 1920 | 1511 |
| miR-152-3p | 1854 | 2393 | 0 | 0 | 1209 | 1746 |
| miR-15a-5p | 9268 | 11,252 | 8791 | 6577 | 10,976 | 7963 |
| miR-15b-5p | 10,225 | 11,817 | 7676 | 4862 | 12,178 | 8735 |
| miR-16-5p | 23,877 | 29,760 | 24,744 | 19,313 | 33,102 | 24,885 |
| miR-17-5p | 3092 | 3465 | 3345 | 2828 | 3773 | 3677 |
| miR-181a-3p | 270 | 0 | 0 | 0 | 0 | 0 |
| miR-181a-5p | 2155 | 1557 | 1241 | 1021 | 1466 | 1414 |
| miR-181b-5p | 944 | 634 | 0 | 0 | 0 | 0 |
| miR-181d-5p | 714 | 473 | 0 | 0 | 0 | 0 |
| miR-183-5p | 527 | 365 | 0 | 0 | 0 | 0 |
| miR-185-5p | 610 | 540 | 0 | 632 | 0 | 0 |
| miR-186-5p | 1164 | 1372 | 1963 | 1844 | 1696 | 1709 |
| miR-18a-5p | 437 | 457 | 0 | 0 | 0 | 0 |
| miR-191-5p | 3574 | 3677 | 3092 | 2965 | 3710 | 4040 |
| miR-193a-3p | 3563 | 2401 | 0 | 587 | 2061 | 2227 |
| miR-195-5p | 374 | 804 | 0 | 0 | 1055 | 0 |
| miR-196a-5p | 5952 | 4730 | 1297 | 602 | 3301 | 3194 |
| miR-196b-3p | 815 | 223 | 0 | 0 | 0 | 0 |
| miR-196b-5p | 2520 | 2101 | 0 | 0 | 1290 | 0 |
| miR-199a-5p | 15,996 | 17,854 | 6145 | 3584 | 13,123 | 13,706 |
| miR-19a-3p | 1182 | 1504 | 2363 | 2298 | 2326 | 1862 |
| miR-19b-3p | 6114 | 5510 | 6119 | 5431 | 6073 | 5334 |
| miR-204-3p | 992 | 285 | 0 | 578 | 0 | 0 |
| miR-205-5p | 0 | 117 | 0 | 0 | 1655 | 0 |
| miR-20a-5p | 1715 | 2219 | 2308 | 1860 | 2061 | 2192 |
| miR-20b-5p | 1504 | 1628 | 1924 | 1286 | 2043 | 1913 |
| miR-210-3p | 7921 | 5347 | 1569 | 1288 | 3041 | 3329 |
| miR-214-5p | 772 | 860 | 0 | 0 | 0 | 0 |
| miR-21-5p | 56,378 | 115,717 | 50,802 | 29,227 | 84,586 | 76,286 |
| miR-218-5p | 1364 | 3301 | 0 | 0 | 942 | 1318 |
| miR-221-3p | 60,280 | 36,507 | 17,469 | 9313 | 37,071 | 32,350 |
| miR-222-3p | 45,874 | 33,045 | 13,637 | 6941 | 31,186 | 26,064 |
| miR-223-3p | 0 | 0 | 10,435 | 11,464 | 3899 | 3621 |
| miR-22-3p | 30,464 | 35,897 | 9711 | 5829 | 19,711 | 16,146 |
| miR-22-5p | 831 | 857 | 0 | 0 | 0 | 0 |
| miR-23a-3p | 14,530 | 15,288 | 9451 | 6039 | 17,685 | 18,471 |
| miR-23a-5p | 1364 | 318 | 0 | 0 | 1541 | 1513 |
| miR-23b-3p | 9879 | 11,605 | 7103 | 4374 | 12,388 | 13,316 |
| miR-24-3p | 14,019 | 13,811 | 5365 | 3360 | 10,476 | 10,575 |
| miR-25-3p | 3410 | 3478 | 4018 | 4686 | 3312 | 3240 |
| miR-26a-5p | 14,943 | 24,472 | 11,473 | 8941 | 16,360 | 14,933 |
| miR-26b-5p | 4340 | 5251 | 5304 | 4160 | 5172 | 4732 |
| miR-27a-3p | 9509 | 9645 | 11,580 | 5186 | 18,358 | 13,643 |
| miR-27a-5p | 542 | 183 | 0 | 0 | 0 | 0 |
| miR-27b-3p | 3745 | 5331 | 5673 | 2863 | 10,455 | 7796 |
| miR-28-5p | 2498 | 2798 | 0 | 0 | 1707 | 1785 |
| miR-29a-3p | 32,286 | 36,765 | 14,087 | 7669 | 29,072 | 29,852 |
| miR-29a-5p | 442 | 545 | 0 | 0 | 0 | 0 |
| miR-29b-3p | 33,224 | 43,943 | 15,536 | 7714 | 27,775 | 26,096 |
| miR-29c-3p | 1299 | 3281 | 1722 | 1594 | 3460 | 3008 |
| miR-301a-3p | 1613 | 1329 | 1057 | 687 | 1350 | 1342 |
| miR-30a-3p | 431 | 298 | 0 | 0 | 0 | 0 |
| miR-30a-5p | 3415 | 4477 | 2124 | 1653 | 3523 | 3333 |
| miR-30b-5p | 3544 | 4877 | 4024 | 3000 | 5261 | 4684 |
| miR-30c-2-3p | 253 | 122 | 0 | 0 | 0 | 0 |
| miR-30c-5p | 5151 | 7006 | 4198 | 2929 | 5608 | 4956 |
| miR-30d-5p | 4961 | 5492 | 2658 | 1607 | 4626 | 4038 |
| miR-30e-3p | 464 | 299 | 0 | 0 | 0 | 0 |
| miR-30e-5p | 1188 | 1537 | 1222 | 953 | 1537 | 1351 |
| miR-320a | 2177 | 1001 | 0 | 576 | 1707 | 1609 |
| miR-324-5p | 970 | 620 | 0 | 0 | 0 | 0 |
| miR-328-3p | 1955 | 975 | 0 | 1194 | 1092 | 1342 |
| miR-331-3p | 829 | 642 | 0 | 0 | 0 | 0 |
| miR-33a-5p | 2930 | 2128 | 0 | 625 | 1409 | 1453 |
| miR-342-3p | 0 | 154 | 1680 | 1424 | 0 | 0 |
| miR-34a-5p | 2393 | 2012 | 0 | 0 | 1242 | 1193 |
| miR-34c-5p | 8039 | 7379 | 2217 | 1038 | 4598 | 3652 |
| miR-361-5p | 304 | 478 | 0 | 0 | 0 | 0 |
| miR-374b-5p | 685 | 749 | 0 | 0 | 1272 | 0 |
| miR-425-5p | 1256 | 1249 | 1076 | 1122 | 966 | 0 |
| miR-451a | 0 | 0 | 194,289 | 267,727 | 46,810 | 40,211 |
| miR-484 | 6203 | 5410 | 2543 | 1564 | 4918 | 5300 |
| miR-486-5p | 0 | 146 | 3971 | 5677 | 1179 | 0 |
| miR-532-3p | 354 | 254 | 1239 | 2490 | 0 | 1400 |
| miR-532-5p | 251 | 258 | 0 | 0 | 0 | 0 |
| miR-574-3p | 5199 | 3713 | 48,691 | 74,742 | 25,101 | 48,180 |
| miR-574-5p | 20,670 | 18,789 | 180,234 | 217,257 | 78,303 | 149,525 |
| miR-615-3p | 1621 | 944 | 0 | 0 | 1150 | 0 |
| miR-652-3p | 1455 | 1110 | 0 | 860 | 927 | 0 |
| miR-671-5p | 431 | 149 | 0 | 0 | 0 | 0 |
| miR-6766-3p | 3846 | 222 | 0 | 1818 | 0 | 0 |
| miR-7-1-3p | 346 | 307 | 0 | 0 | 0 | 0 |
| miR-744-5p | 254 | 141 | 0 | 0 | 0 | 0 |
| miR-7-5p | 9101 | 10,666 | 3405 | 897 | 6088 | 3271 |
| miR-877-5p | 284 | 0 | 0 | 0 | 0 | 0 |
| miR-92b-3p | 1302 | 1503 | 0 | 866 | 1478 | 1527 |
| miR-93-5p | 3329 | 3218 | 3205 | 2549 | 3023 | 3064 |
| miR-96-5p | 1243 | 965 | 0 | 0 | 0 | 0 |
| miR-99a-5p | 7734 | 8614 | 2852 | 1730 | 5380 | 5383 |
| miR-99b-3p | 550 | 288 | 0 | 0 | 0 | 0 |
| miR-99b-5p | 6388 | 5799 | 2252 | 1342 | 5311 | 4932 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozier, P.; Maumus, M.; Maria, A.T.J.; Toupet, K.; Jorgensen, C.; Guilpain, P.; Noël, D. Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis. Cells 2021, 10, 2727. https://doi.org/10.3390/cells10102727
Rozier P, Maumus M, Maria ATJ, Toupet K, Jorgensen C, Guilpain P, Noël D. Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis. Cells. 2021; 10(10):2727. https://doi.org/10.3390/cells10102727
Chicago/Turabian StyleRozier, Pauline, Marie Maumus, Alexandre Thibault Jacques Maria, Karine Toupet, Christian Jorgensen, Philippe Guilpain, and Danièle Noël. 2021. "Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis" Cells 10, no. 10: 2727. https://doi.org/10.3390/cells10102727