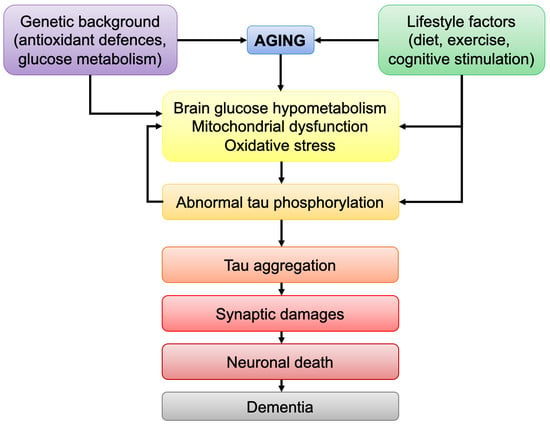
Impairments in Brain Bioenergetics in Aging and Tau Pathology: A Chicken and Egg Situation?
Abstract
:1. Introduction
- (i)
- Primary tauopathies, such as PSP and CBD but also frontotemporal lobar degeneration (FTLD), in which tau plays a central role and mutant forms of tau have been identified [4].
- (ii)
- Secondary tauopathies, such as AD, in which tau also plays a pathological role but other major factors appear to be involved (e.g., amyloid-β accumulation in AD). Although abnormal tau protein is clearly a hallmark of AD, no mutation in the tau-coding gene has been so far linked to the disease, suggesting that other factors are responsible for abnormal tau hyperphosphorylation and aggregation.
2. Brain Bioenergetics and Aging
2.1. Alteration in Glucose Metabolism
- (i)
- (ii)
2.2. Alteration in Mitochondrial Function
- (i)
- Decreased mitochondrial bioenergetics, namely a decrease in the expression/activity of mitochondrial complexes (including complex I, III, IV and V) involved in OxPhos, decreased mitochondrial respiration (OxPhos) and ATP production.
- (ii)
- Defects in mitochondrial dynamics (fusion/fission activity), mitochondrial transport/distribution, and mitophagy. Indeed, mitochondria are very dynamic organelles that constantly fuse and divide to mix their protein, lipid and mitochondrial DNA (mtDNA) content. Therefore, a decrease in mitochondrial quality control (mitochondrial dynamics/mitophagy) would lead to an accumulation of damaged mitochondria, and accumulation of mtDNA mutations. mtDNA encodes subunits of complexes of the electron transport chain (ETC, complex I, III, IV). Evidence shows that the accumulation of mtDNA mutations over time is a central mechanism driving aging and age-related diseases, as they may lead to decreased ETC activity and impaired mitochondrial respiration [1,18].
2.3. Increase of Oxidative Damages
- (i)
- A decrease in antioxidant defenses in the brain, namely a decrease in superoxide dismutase activity, in GSH content and GSH/GSSG ratio (reduced/oxidized glutathione).
- (ii)
- An increase in oxidative damages, including increased free-radical level, DNA oxidative damages and lipid peroxidation.
3. Abnormal Tau and Bioenergetic Impairments
3.1. Abnormal Tau and Mitochondrial Dysfunction
- (i)
- (ii)
- (iii)
- Mitophagy: abnormal tau was shown to inhibit mitophagy by interacting with Parkin, a key protein involved in the mitochondria quality control process [23].
- (iv)
3.2. Abnormal Tau and Brain Glucose Metabolism
4. Age-Related Bioenergetic Impairments in the Brain: A Trigger for Tau Pathology?
4.1. Impaired Glucose Metabolism and Tau Pathology
4.2. Effects of Increased ROS Levels and Tau Pathology
5. Lifestyle Factors Influencing Brain Metabolism and Tau Pathology
5.1. Diet
5.2. Physical Exercice
- (i)
- Blood flow, which improves nutrient import into the brain (Figure 3).
- (ii)
- Lactate levels in the blood due to muscular activity. Lactate can then be transported into the brain and directly used by neurons as a bioenergetic substrate for the TCA cycle and OxPhos.
- (iii)
- Brain-derived neurotrophic factor (BDNF) levels, and other proteins involved in mitochondrial biogenesis, which increases synaptic plasticity, learning and memory, as well as improves resistance to stress and neurodegeneration (reviewed in [2]).
5.3. Intellectual Challenge
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camandola, S.; Mattson, M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic Energy Use and Supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Rojas, C.; Cabezas-Opazo, F.; Deaton, C.A.; Vergara, E.H.; Johnson, G.V.W.; Quintanilla, R.A. It’s all about tau. Prog. Neurobiol. 2019, 175, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau Associates With Changes in Its Function Beyond Microtubule Stability. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Sebastián-Serrano, Á.; de Diego-García, L.; Díaz-Hernández, M. The Neurotoxic Role of Extracellular Tau Protein. Int. J. Mol. Sci. 2018, 19, 998. [Google Scholar] [CrossRef] [Green Version]
- Szabo, L.; Eckert, A.; Grimm, A. Insights into Disease-Associated Tau Impact on Mitochondria. Int. J. Mol. Sci. 2020, 21, 6344. [Google Scholar] [CrossRef] [PubMed]
- Mason, S. Lactate Shuttles in Neuroenergetics—Homeostasis, Allostasis and Beyond. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Mosconi, L.; De Santi, S.; Li, J.; Tsui, W.H.; Li, Y.; Boppana, M.; Laska, E.; Rusinek, H.; de Leon, M.J. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol. Aging 2008, 29, 676–692. [Google Scholar] [CrossRef] [Green Version]
- Goyal, M.S.; Vlassenko, A.G.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.S.; Morris, J.C.; Raichle, M.E. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab. 2017, 26, 353–360.e353. [Google Scholar] [CrossRef]
- Bentourkia, M.; Bol, A.; Ivanoiu, A.; Labar, D.; Sibomana, M.; Coppens, A.; Michel, C.; Cosnard, G.; De Volder, A.G. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: Effect of aging. J. Neurol. Sci. 2000, 181, 19–28. [Google Scholar] [CrossRef]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement 2018, 14, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 943–972. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Yao, J.; Rettberg, J.R.; Chen, S.; Brinton, R.D. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: Implication for bioenergetic intervention. PLoS ONE 2013, 8, e79977. [Google Scholar] [CrossRef] [Green Version]
- Iwangoff, P.; Armbruster, R.; Enz, A.; Meier-Ruge, W. Glycolytic enzymes from human autoptic brain cortex: Normal aged and demented cases. Mech. Ageing Dev. 1980, 14, 203–209. [Google Scholar] [CrossRef]
- Lee, K.Y.; Yoo, D.Y.; Jung, H.Y.; Baek, L.; Lee, H.; Kwon, H.J.; Chung, J.Y.; Kang, S.H.; Kim, D.W.; Hwang, I.K.; et al. Decrease in glucose transporter 1 levels and translocation of glucose transporter 3 in the dentate gyrus of C57BL/6 mice and gerbils with aging. Lab. Anim. Res. 2018, 34. [Google Scholar] [CrossRef] [Green Version]
- DeBalsi, K.L.; Hoff, K.E.; Copeland, W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017, 33, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Eckert, A.; Nisbet, R.; Grimm, A.; Götz, J. March separate, strike together—Role of phosphorylated TAU in mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 1258–1266. [Google Scholar] [CrossRef] [Green Version]
- DuBoff, B.; Götz, J.; Feany, M.B. Tau Promotes Neurodegeneration via DRP1 Mislocalization In Vivo. Neuron 2012, 75, 618–632. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-C.; Hu, Y.; Wang, Z.-h.; Luo, Y.; Zhang, Y.; Liu, X.-P.; Feng, Q.; Wang, Q.; Ye, K.; Liu, G.-P.; et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Cummins, N.; Tweedie, A.; Zuryn, S.; Bertran-Gonzalez, J.; Götz, J. Disease-associated tau impairs mitophagy by inhibiting Parkin translocation to mitochondria. EMBO J. 2018, 38. [Google Scholar] [CrossRef]
- David, D.C.; Hauptmann, S.; Scherping, I.; Schuessel, K.; Keil, U.; Rizzu, P.; Ravid, R.; Dröse, S.; Brandt, U.; Müller, W.E.; et al. Proteomic and Functional Analyses Reveal a Mitochondrial Dysfunction in P301L Tau Transgenic Mice. J. Biol. Chem. 2005, 280, 23802–23814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, A.; Biliouris, E.E.; Lang, U.E.; Götz, J.; Mensah-Nyagan, A.G.; Eckert, A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell. Mol. Life Sci. 2015, 73, 201–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Drose, S.; Brandt, U.; et al. Amyloid- and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Nat. Acad. Sci. USA 2009, 106, 20057–20062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.L.; Eckert, A.; Rhein, V.; Mai, S.; Haase, W.; Reichert, A.S.; Jendrach, M.; Müller, W.E.; Leuner, K. A New Link to Mitochondrial Impairment in Tauopathies. Mol. Neurobiol. 2012, 46, 205–216. [Google Scholar] [CrossRef]
- Dukart, J.; Kherif, F.; Mueller, K.; Adaszewski, S.; Schroeter, M.L.; Frackowiak, R.S.; Draganski, B.; Alzheimer’s Disease Neuroimaging, I. Generative FDG-PET and MRI model of aging and disease progression in Alzheimer’s disease. PLoS Comput. Biol. 2013, 9, e1002987. [Google Scholar] [CrossRef] [Green Version]
- Dukart, J.; Mueller, K.; Villringer, A.; Kherif, F.; Draganski, B.; Frackowiak, R.; Schroeter, M.L. Relationship between imaging biomarkers, age, progression and symptom severity in Alzheimer’s disease. NeuroImage Clin. 2013, 3, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Schöll, M.; Maass, A.; Mattsson, N.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Jagust, W. Biomarkers for tau pathology. Mol. Cell. Neurosci. 2019, 97, 18–33. [Google Scholar] [CrossRef]
- Beyer, L.; Meyer-Wilmes, J.; Schönecker, S.; Schnabel, J.; Brendel, E.; Prix, C.; Nübling, G.; Unterrainer, M.; Albert, N.L.; Pogarell, O.; et al. Clinical Routine FDG-PET Imaging of Suspected Progressive Supranuclear Palsy and Corticobasal Degeneration: A Gatekeeper for Subsequent Tau-PET Imaging? Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghel, V.; Tripathi, M.; Parida, G.; Gupta, R.; Yadav, S.; Kumar, P.; Dey, A.B.; Damle, N.A.; Kumar, R.; Bal, C. In Vivo Assessment of Tau Deposition in Alzheimer Disease and Assessing Its Relationship to Regional Brain Glucose Metabolism and Cognition. Clin. Nucl. Med. 2019, 44, e597–e601. [Google Scholar] [CrossRef] [PubMed]
- Chiaravalloti, A.; Barbagallo, G.; Ricci, M.; Martorana, A.; Ursini, F.; Sannino, P.; Karalis, G.; Schillaci, O. Brain metabolic correlates of CSF Tau protein in a large cohort of Alzheimer’s disease patients: A CSF and FDG PET study. Brain Res. 2018, 1678, 116–122. [Google Scholar] [CrossRef]
- Vlassenko, A.G.; Gordon, B.A.; Goyal, M.S.; Su, Y.; Blazey, T.M.; Durbin, T.J.; Couture, L.E.; Christensen, J.J.; Jafri, H.; Morris, J.C.; et al. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol. Aging 2018, 67, 95–98. [Google Scholar] [CrossRef]
- Ou, Y.-N.; Xu, W.; Li, J.-Q.; Guo, Y.; Cui, M.; Chen, K.-L.; Huang, Y.-Y.; Dong, Q.; Tan, L.; Yu, J.-T. FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: A longitudinal study. Alzheimer Res. Ther. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Ottoy, J.; Niemantsverdriet, E.; Verhaeghe, J.; De Roeck, E.; Struyfs, H.; Somers, C.; Wyffels, L.; Ceyssens, S.; Van Mossevelde, S.; Van den Bossche, T.; et al. Association of short-term cognitive decline and MCI-to-AD dementia conversion with CSF, MRI, amyloid- and 18F-FDG-PET imaging. NeuroImage Clin. 2019, 22. [Google Scholar] [CrossRef]
- Chiotis, K.; Saint-Aubert, L.; Rodriguez-Vieitez, E.; Leuzy, A.; Almkvist, O.; Savitcheva, I.; Jonasson, M.; Lubberink, M.; Wall, A.; Antoni, G.; et al. Longitudinal changes of tau PET imaging in relation to hypometabolism in prodromal and Alzheimer’s disease dementia. Mol. Psychiatry 2017, 23, 1666–1673. [Google Scholar] [CrossRef]
- Ackley, S.F.; Zimmerman, S.C.; Brenowitz, W.D.; Tchetgen Tchetgen, E.J.; Gold, A.L.; Manly, J.J.; Mayeda, E.R.; Filshtein, T.J.; Power, M.C.; Elahi, F.M.; et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: Instrumental variable meta-analysis. BMJ 2021, 372, n156. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Tredici, K.D.; et al. Correlation of Alzheimer Disease Neuropathologic Changes With Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Jagust, W.J.; Li, L.; Lockhart, S.N.; Adams, J.N. Relationships Between Tau and Glucose Metabolism Reflect Alzheimer’s Disease Pathology in Cognitively Normal Older Adults. Cereb. Cortex 2019, 29, 1997–2009. [Google Scholar] [CrossRef]
- Rubinski, A.; Franzmeier, N.; Neitzel, J.; Ewers, M. FDG-PET hypermetabolism is associated with higher tau-PET in mild cognitive impairment at low amyloid-PET levels. Alzheimer Res. Ther. 2020, 12. [Google Scholar] [CrossRef]
- Demetrius, L.A.; Magistretti, P.J.; Pellerin, L. Alzheimer’s disease: The amyloid hypothesis and the Inverse Warburg effect. Front. Physiol. 2014, 5, 522. [Google Scholar] [CrossRef] [Green Version]
- Demetrius, L.A.; Simon, D.K. An inverse-Warburg effect and the origin of Alzheimer’s disease. Biogerontology 2012, 13, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Ng, G.; Tan, E.K.; Liao, P.; Kandiah, N.; Zeng, L. Chronic cerebral hypoperfusion enhances Tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Gu, J.-h.; Dai, C.-l.; Liu, Q.; Iqbal, K.; Liu, F.; Gong, C.-X. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.K.; Simoes, S.; Baena-Caldas, G.P.; Lao, P.J.; Kothiya, M.; Igwe, K.C.; Chesebro, A.G.; Houck, A.L.; Pedraza, L.; Hernández, A.I.; et al. Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain Commun. 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, K.; Pereira, J.B.; Berron, D.; Vogel, J.; Ingala, S.; Strandberg, O.T.; Janelidze, S.; Barkhof, F.; Pfeuffer, J.; Knutsson, L.; et al. Cerebral hypoperfusion is a late pathological event in the course of Alzheimer’s disease. medRxiv 2021. medRxiv: 2021.07.02.21259911. [Google Scholar] [CrossRef]
- Lauretti, E.; Praticò, D. Glucose deprivation increases tau phosphorylation via P 38 mitogen-activated protein kinase. Aging Cell 2015, 14, 1067–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauretti, E.; Li, J.G.; Di Meco, A.; Praticò, D. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl. Psychiatry 2017, 7, e1020. [Google Scholar] [CrossRef] [PubMed]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxidative Med. Cell. Longev. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13. [Google Scholar] [CrossRef]
- Ibáñez-Salazar, A.; Bañuelos-Hernández, B.; Rodríguez-Leyva, I.; Chi-Ahumada, E.; Monreal-Escalante, E.; Jiménez-Capdeville, M.E.; Rosales-Mendoza, S. Oxidative Stress Modifies the Levels and Phosphorylation State of Tau Protein in Human Fibroblasts. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Adlard, P.A.; Morten, K.; Johnson, F.; Golden, T.R.; Hinerfeld, D.; Schilling, B.; Mavros, C.; Masters, C.L.; Volitakis, I.; et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE 2007, 2, e536. [Google Scholar] [CrossRef]
- Dumont, M.; Stack, C.; Elipenahli, C.; Jainuddin, S.; Gerges, M.; Starkova, N.N.; Yang, L.; Starkov, A.A.; Beal, F. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. FASEB J. 2011, 25, 4063–4072. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Wang, X.; Lee, H.-g.; Tabaton, M.; Perry, G.; Smith, M.A.; Zhu, X. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci. Lett. 2010, 468, 267–271. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, Y.; Yu, G.; Shu, X.; Ge, H.; Zeng, K.; Wang, J.; Wang, X. Cleavage of GSK-3β by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3β activity induced by H2O2. J. Neurochem. 2013, 126, 234–242. [Google Scholar] [CrossRef]
- Dias-Santagata, D.; Fulga, T.A.; Duttaroy, A.; Feany, M.B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Investig. 2007, 117, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, Y.; Mishra, A.; Bacon, E.; Yin, F.; Brinton, R. Midlife Chronological and Endocrinological Transitions in Brain Metabolism: System Biology Basis for Increased Alzheimer’s Risk in Female Brain. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Halagappa, V.K.M.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Bergman, C.; Lee, J.-H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlow, M.L.; Joly-Amado, A.; Azam, S.; Elza, M.; Selenica, M.-L.; Pappas, C.; Small, B.; Engelman, R.; Gordon, M.N.; Morgan, D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Rese. 2014, 271, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Benner, L.; D’Agostino, D.; Gordon, M.N.; Morgan, D. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS ONE 2013, 8, e75713. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhu, H.; Taheri, S.; Mondy, W.; Perry, S.; Kindy, M.S. Impact of nutrition on inflammation, tauopathy, and behavioral outcomes from chronic traumatic encephalopathy. J. Neuroinflamm. 2018, 15, 277. [Google Scholar] [CrossRef]
- Ohia-Nwoko, O.; Montazari, S.; Lau, Y.-S.; Eriksen, J.L. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Mol. Neurodegener. 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratuze, M.; Julien, J.; Morin, F.; Marette, A.; Planel, E. Differential effects of voluntary treadmill exercise and caloric restriction on tau pathogenesis in a mouse model of Alzheimer’s disease-like tau pathology fed with Western diet. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 79, 452–461. [Google Scholar] [CrossRef]
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.-J.; Laurent, C.; Lestavel, S.; Figeac, M.; Sultan, A.; Troquier, L.; Leboucher, A.; Caillierez, R.; et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol. Dis. 2011, 43, 486–494. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Tucker, D.; Dong, Y.A.N.; Zhu, L.; Duan, R.U.I.; Liu, T.C.-Y.; Zhang, Q. Beneficial Effects of Exercise Pretreatment in a Sporadic Alzheimer’s Rat Model. Med. Sci. Sports Exerc. 2018, 50, 945–956. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Koo, J.-H.; Cho, J.-Y.; Kang, E.-B. Neuroprotective effect of treadmill exercise against blunted brain insulin signaling, NADPH oxidase, and Tau hyperphosphorylation in rats fed a high-fat diet. Brain Res. Bull. 2018, 142, 374–383. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, J.M.T.; Yan, T.; Zhang, Y.; Chen, Y.; Chang, R.C.C.; Wong, G.T.C. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice. J. Neuroinflamm. 2020, 17. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.S.; Xu, P.; Pigino, G.; Brady, S.T.; Larson, J.; Lazarov, O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1ΔE9 mice. FASEB J. 2010, 24, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Izquierdo, V.; Otero, E.; Puigoriol-Illamola, D.; Corpas, R.; Sanfeliu, C.; Ortuño-Sahagún, D.; Pallàs, M. Environmental Enrichment Improves Cognitive Deficits, AD Hallmarks and Epigenetic Alterations Presented in 5xFAD Mouse Model. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiani-Cohen, I.; Lourbopoulos, A.; Haber, E.; Rozenstein-Tsalkovich, L.; Abramsky, O.; Grigoriadis, N.; Rosenmann, H. Moderate Environmental Enrichment Mitigates Tauopathy in a Neurofibrillary Tangle Mouse Model. J. Neuropathol. Exp. Neurol. 2011, 70, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Selvi, Y.; Gergerlioglu, H.S.; Akbaba, N.; Oz, M.; Kandeger, A.; Demir, E.A.; Yerlikaya, F.H.; Nurullahoglu-Atalik, K.E. Impact of enriched environment on production of tau, amyloid precursor protein and, amyloid-β peptide in high-fat and high-sucrose-fed rats. Acta Neuropsychiatr. 2016, 29, 291–298. [Google Scholar] [CrossRef]
- Huber, B.R.; Alosco, M.L.; Stein, T.D.; McKee, A.C. Potential Long-Term Consequences of Concussive and Subconcussive Injury. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.J.; Noh, Y.-H.; Lee, J.-H.; Jin, M.; Kim, J.-S.; Han, S.-D. Diet control to achieve euglycaemia induces tau hyperphosphorylation via AMPK activation in the hippocampus of diabetic rats. Food Funct. 2020, 11, 339–346. [Google Scholar] [CrossRef]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Solingapuram Sai, K.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J.; et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: A pilot study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.; Rainey-Smith, S.R. Exploring the relationship between physical activity, beta-amyloid and tau: A narrative review. Ageing Res. Rev. 2019, 50, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Rainey-Smith, S.R.; Dore, V.; Peiffer, J.J.; Burnham, S.C.; Laws, S.M.; Taddei, K.; Ames, D.; Masters, C.L.; Rowe, C.C.; et al. Self-Reported Physical Activity is Associated with Tau Burden Measured by Positron Emission Tomography. J. Alzheimer Dis. 2018, 63, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, K.S.; Gjerum, L.; Waldemar, G.; Hasselbalch, S.G. Physical Activity as a Moderator of Alzheimer Pathology: A Systematic Review of Observational Studies. Curr. Alzheimer Res. 2019, 16, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Trebbastoni, A.; Imbriano, L.; Podda, L.; Rendace, L.; Sacchett, M.L.; Campanelli, A.; D’Antonio, F.; de Lena, C. Cognitive Training in Patients with Alzheimer’s Disease: Findings of a 12-month Randomized Controlled Trial. Curr. Alzheimer Res. 2018, 15, 452–461. [Google Scholar] [CrossRef]
- Carrion, C.; Folkvord, F.; Anastasiadou, D.; Aymerich, M. Cognitive Therapy for Dementia Patients: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2018, 46, 1–26. [Google Scholar] [CrossRef]
- Hoenig, M.C.; Bischof, G.N.; Hammes, J.; Faber, J.; Fliessbach, K.; van Eimeren, T.; Drzezga, A. Tau pathology and cognitive reserve in Alzheimer’s disease. Neurobiol. Aging 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]




| Model | Methods | Main Effects of Modified Diet/Physical Exercise/Cognitive Stimulation | Ref. |
|---|---|---|---|
| 3xTgAD mice | Calorie restriction (CR) and intermittent fasting (IF) beginning at 3 months of age | Suppression of tau pathology with CR, but not IF, in the hippocampus of old miceCR and IF ameliorate age-related cognitive deficits | [62] |
| Tg4510 mice | 12-weeks caloric restriction (CR) | No effet of CR on tau pathologyImprovment of short-term memory and contextual memory (trend), but not spatial memory | [64] |
| 3xTgAD mice | 8-months ketone ester-based diet | Reduced levels of hyperphosphorylated tau deposition in the CA1 and CA3 regions of the hippocampus, amygdala, and cortexSlight improvement of congnitive function (learning and memory) | [63] |
| Tg4510 mice | 3-months ketogenic diet (KD) | Enhancement of motor performanceNo effect of KD on cognitive function and tau pathology | [65] |
| h-tau mice with repetitive mild traumatic brain injuries (rmTBI = controlled, repetitive closed head impacts) | NutriFusion diets * for 2 months prior to the rmTBI | Prevention of tau pathologyImproved behavioral outcomes after rmTBI, including learning and memory | [66] |
| P301S tau mice | 12-weeks of forced treadmill exercise | Significant reduction in full-length and hyperphosphorylated tau (spinal cord and hippocampus)Reductin of insoluble tau in the spinal cordImprovment of locomotor and exploratory activityNo significant attenuation of neuronal death in the hippocampus | [67] |
| h-tau mice | 2-months voluntary physical exercise (running wheel) and caloric restriction in h-tau mice under high caloric diet (obese mice) | Reduction of tau phosphorylation with physical activityIncreased tau aggregation with caloric restriction in the brain of obese mice | [68] |
| THY-Tau22 mice | 9-months voluntary physical exercise (running wheel) | Prevention of memory deficits by physical exerciseDecreased tau pathology in the hippocampus | [69] |
| streptozotocin (STZ)-induced sporadic AD rats | Swimming exercise training for 4 weeks before STZ injection | Decrease of STZ-induced tau hyperphosphorylation and oxidative damagesPrevention of STZ-induced cognitive dysfunction and synaptic loss/cell death in the hippocampal CA1 region | [70] |
| Obese Sprague-Dawley rats (high-fat diet (HFD) for 20 weeks) | 8-weeks treadmill exercise (progressively increasing load intensity) | Decreased hyperphosphorylation and aggregation of Tau proteinImprovment of cognitive function (learning and memory) | [71] |
| 3xTgAD mice | Short-term resistance training (climbing up a 1-m ladder with a progressively heavier weight loading) | Decreased total and hyperphosphorylated tau in the frontal cortex and hippocampusImprovement of cognitive performance | [72] |
| APPswe/PS1ΔE9 mice | 3 h/day environmental enrichment (EE) for 1 or 2 months = mice transferred in enlarged cages with running wheels, colored tunnels, visual stimulating toys. Objects in the cage were repositioned for novel stimulation every day | Enhancement of neurogenesisSignificant reduction in levels of hyperphosphorylated tau in the hippocampus and cortex Enhancement of hippocampal long-term potentiation | [73] |
| 5xFAD mice | 8 weeks in EE conditions = cages in which plastic tubes, plastic dolls or toys were added, extracted, or changed every week | Reduced cognitive deficitsIncreased neuroplasticityDecrease of tau phosphorylation | [74] |
| E257K/P301S-Tau (DM-Tau Tg) mice | Mice housed for 9 months in EE versus regular environment cages.EE = every week, mice are transferred to new enlarged cages equipped with a running whell and differently shaped objects (tunnels, boxes, cubes, balls, ladder, labyrinth) | Reduced neurofibrillary tangle (NFT) burdenIncreased neurogenesisIncrease in brain-derived neurotrophic factor (BDNF) levelsTrend toward improvement in cognitive tasks | [75] |
| High-fat, high-sucrose fed rats | Rats housed in EE = cages containing objects such as toys, tunnels, running wheels, stairs and platforms. The EE design was changed twice a week | Normalization of tau protein level to the control group (normal diet) | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimm, A. Impairments in Brain Bioenergetics in Aging and Tau Pathology: A Chicken and Egg Situation? Cells 2021, 10, 2531. https://doi.org/10.3390/cells10102531
Grimm A. Impairments in Brain Bioenergetics in Aging and Tau Pathology: A Chicken and Egg Situation? Cells. 2021; 10(10):2531. https://doi.org/10.3390/cells10102531
Chicago/Turabian StyleGrimm, Amandine. 2021. "Impairments in Brain Bioenergetics in Aging and Tau Pathology: A Chicken and Egg Situation?" Cells 10, no. 10: 2531. https://doi.org/10.3390/cells10102531