Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells
Abstract
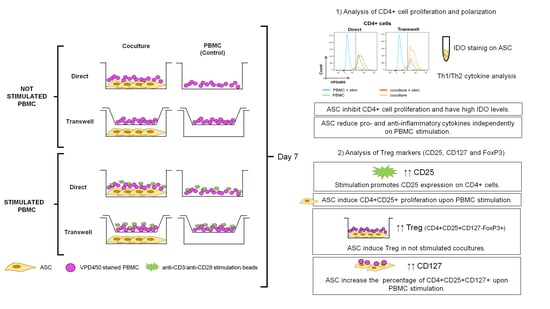
:1. Introduction
2. Materials and Methods
2.1. Generation of ASC and PBMC
2.2. PBMC Staining before Coculture
2.3. PBMC:ASC Coculture
2.4. Flow Cytometry
Analysis of Division Index
2.5. Cytokine Analysis
2.5.1. LEGENDplex™
2.5.2. ELISA
2.5.3. Kynurenine Detection
2.5.4. IDO Staining
2.6. Statistics
3. Results
3.1. ASC Abrogate CD4+ Cell Proliferation When Cocultured with Stimulated PBMC
3.2. Cocultures Reduce Th Cytokine Secretion While Inducing TGF-β
3.3. CD4+CD25+ Cells Are Induced by Stimulation and Expanded by ASC
3.4. ASC Induce Treg and CD127 Upon Stimulation
3.5. ASC-Mediated Treg Induction Originate from a Variation in the Proportion of CD127/FoxP3 Expression within the CD4+CD25+ Subpopulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, M.A.; Gonzalez-Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009, 136, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Zhuansun, Y.; Liu, G.; Chen, R.; Li, J.; Ran, P. Mesenchymal stem cells suppress T cells by inducing apoptosis and through PD-1/B7-H1 interactions. Immunol. Lett. 2014, 162, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 6. [Google Scholar] [CrossRef]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed. Res. Int. 2014, 2014, 216806. [Google Scholar] [CrossRef] [Green Version]
- Meisel, R.; Zibert, A.; Laryea, M.; Gobel, U.; Daubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [Green Version]
- Gur-Wahnon, D.; Borovsky, Z.; Beyth, S.; Liebergall, M.; Rachmilewitz, J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp. Hematol. 2007, 35, 426–433. [Google Scholar] [CrossRef]
- Liang, C.; Jiang, E.; Yao, J.; Wang, M.; Chen, S.; Zhou, Z.; Zhai, W.; Ma, Q.; Feng, S.; Han, M. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology 2018, 23, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffi, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrión, F.; Nova, E.; Luz, P.; Apablaza, F.; Figueroa, F. Opposing effect of mesenchymal stem cells on Th1 and Th17 cell polarization according to the state of CD4+ T cell activation. Immunol. Lett. 2011, 135, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Ianni, M.; Del Papa, B.; De Ioanni, M.; Moretti, L.; Bonifacio, E.; Cecchini, D.; Sportoletti, P.; Falzetti, F.; Tabilio, A. Mesenchymal cells recruit and regulate T regulatory cells. Exp. Hematol. 2008, 36, 309–318. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Flavell, R.A. How diverse--CD4 effector T cells and their functions. J. Mol. Cell. Biol. 2009, 1, 20–36. [Google Scholar] [CrossRef]
- Corthay, A. How do regulatory T cells work? Scand J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Bacchetta, R.; Gambineri, E.; Roncarolo, M.G. Role of regulatory T cells and FOXP3 in human diseases. J. Allergy Clin. Immunol. 2007, 120, 227–235, quiz 236-227. [Google Scholar] [CrossRef]
- Dunham, R.M.; Cervasi, B.; Brenchley, J.M.; Albrecht, H.; Weintrob, A.; Sumpter, B.; Engram, J.; Gordon, S.; Klatt, N.R.; Frank, I.; et al. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J. Immunol. 2008, 180, 5582–5592. [Google Scholar] [CrossRef] [Green Version]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Engela, A.U.; Hoogduijn, M.J.; Boer, K.; Litjens, N.H.; Betjes, M.G.; Weimar, W.; Baan, C.C. Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin. Exp. Immunol. 2013, 173, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-Cell Generation and Kidney Allograft Tolerance Induced by Mesenchymal Stem Cells Associated With Indoleamine 2,3-Dioxygenase Expression. Transplantation 2010, 90, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Azzollini, N.; Cassis, P.; Imberti, B.; Morigi, M.; Cugini, D.; Cavinato, R.A.; Todeschini, M.; Solini, S.; Sonzogni, A.; et al. Pretransplant Infusion of Mesenchymal Stem Cells Prolongs the Survival of a Semiallogeneic Heart Transplant through the Generation of Regulatory T Cells. J. Immunol. 2008, 181, 3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattar, P.; Bieback, K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front Immunol. 2015, 6, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najar, M.; Raicevic, G.; Boufker, H.I.; Fayyad Kazan, H.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010, 264, 171–179. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Götherström, C.; Seidel, C.; Sundberg, B.; Sundin, M.; Rosendahl, K.; Tammik, C.; Ringdén, O. Mesenchymal Stem Cells Inhibit the Expression of CD25 (Interleukin-2 Receptor) and CD38 on Phytohaemagglutinin-Activated Lymphocytes. Scand. J. Immunol. 2004, 60, 307–315. [Google Scholar] [CrossRef]
- Yoo, H.S.; Lee, K.; Na, K.; Zhang, Y.X.; Lim, H.J.; Yi, T.; Song, S.U.; Jeon, M.S. Mesenchymal stromal cells inhibit CD25 expression via the mTOR pathway to potentiate T-cell suppression. Cell Death Dis. 2017, 8, e2632. [Google Scholar] [CrossRef]
- Bieback, K.; Hecker, A.; Schlechter, T.; Hofmann, I.; Brousos, N.; Redmer, T.; Besser, D.; Klüter, H.; Müller, A.M.; Becker, M. Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy 2012, 14, 570–583. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Uhlig, S.; Elvers-Hornung, S.; Kluter, H.; Bieback, K. Human Adipose Tissue-Derived Stromal Cells Suppress Human, but Not Murine Lymphocyte Proliferation, via Indoleamine 2,3-Dioxygenase Activity. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.I.; Minskaia, E.; Fernandes-Platzgummer, A.; Vieira, A.I.S.; da Silva, C.L.; Cabral, J.M.S.; Lacerda, J.F. Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro. Stem Cells 2020, 38, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Shatrova, A.N.; Mityushova, E.V.; Vassilieva, I.O.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; Marakhova, I.I. Time-Dependent Regulation of IL-2R α-Chain (CD25) Expression by TCR Signal Strength and IL-2-Induced STAT5 Signaling in Activated Human Blood T Lymphocytes. PLoS ONE 2016, 11, e0167215. [Google Scholar] [CrossRef] [Green Version]
- Cuerquis, J.; Romieu-Mourez, R.; Francois, M.; Routy, J.P.; Young, Y.K.; Zhao, J.; Eliopoulos, N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: Effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy 2014, 16, 191–202. [Google Scholar] [CrossRef] [Green Version]
- English, K.; French, A.; Wood, K.J. Mesenchymal stromal cells: Facilitators of successful transplantation? Cell Stem Cell 2010, 7, 431–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crop, M.J.; Baan, C.C.; Korevaar, S.S.; Ijzermans, J.N.M.; Weimar, W.; Hoogduijn, M.J. Human Adipose Tissue-Derived Mesenchymal Stem Cells Induce Explosive T-Cell Proliferation. Stem Cells Dev. 2010, 19, 11. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 7. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357. [Google Scholar] [CrossRef] [Green Version]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, Y.; Lin, X.; Liang, H.; Yang, D.; Zhang, X.; Ke, J.; Xiao, J.; Chen, Z.; Chen, W.; Zhang, X.; et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells in Parkinson’s Disease: Inhibition of T Helper 17 Cell Differentiation and Regulation of Immune Balance Towards a Regulatory T Cell Phenotype. Clin. Interv. Aging 2020, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, H.; Chen, L.; Fang, N.; Chen, H.; Song, G.; Yu, L.; Zhang, Q.; Zhang, T. Effect of peripheral blood-derived mesenchymal stem cells on macrophage polarization and Th17/Treg balance in vitro. Regen 2020, 14, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Lim, J.H.; Chung, C.J.; Kang, T.J.; Kim, T.Y.; Kim, Y.S.; Roh, T.S.; Lew, D.H. Immune Tolerance of Human Dental Pulp-Derived Mesenchymal Stem Cells Mediated by CD4⁺CD25⁺FoxP3⁺ Regulatory T-Cells and Induced by TGF-β1 and IL-10. Yonsei Med. J. 2017, 58, 1031–1039. [Google Scholar] [CrossRef]
- Rodríguez-Perea, A.L.; Arcia, E.D.; Rueda, C.M.; Velilla, P.A. Phenotypical characterization of regulatory T cells in humans and rodents. Clin. Exp. Immunol. 2016, 185, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. The dichotomous role of IL-2: Tolerance versus immunity. Trends Immunol. 2006, 27, 109–111. [Google Scholar] [CrossRef]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef]
- Maccario, R.; Podesta, M.; Moretta, A.; Cometa, A.; Comoli, P.; Montagna, D.; Daudt, L.; Ibatici, A.; Piaggio, G.; Pozzi, S.; et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005, 90, 516. [Google Scholar]
- Narsale, A.; Moya, R.; Davies, J.D. Human CD4(+) CD25(+) CD127(hi) cells and the Th1/Th2 phenotype. Clin. Immunol. 2018, 188, 103–112. [Google Scholar] [CrossRef]
- Kordelas, L.; Schwich, E.; Dittrich, R.; Horn, P.A.; Beelen, D.W.; Borger, V.; Giebel, B.; Rebmann, V. Individual Immune-Modulatory Capabilities of MSC-Derived Extracellular Vesicle (EV) Preparations and Recipient-Dependent Responsiveness. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Guler, A.; Lopez Venegas, M.; Adankwah, E.; Mayatepek, E.; Nausch, N.; Jacobsen, M. Suppressor of cytokine signalling 3 is crucial for interleukin-7 receptor re-expression after T-cell activation and interleukin-7 dependent proliferation. Eur. J. Immunol. 2020, 50, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Dooms, H.; Wolslegel, K.; Lin, P.; Abbas, A.K. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J. Exp. Med. 2007, 204, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemoto, Y.; Kanai, T.; Takahara, M.; Oshima, S.; Nakamura, T.; Okamoto, R.; Tsuchiya, K.; Watanabe, M. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut 2013, 62, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, V.; D’Anneo, A.; Phillips, B.; Engman, C.; Harnaha, J.; Lakomy, R.; Styche, A.; Trucco, M.; Giannoukakis, N. Interleukin-7 matures suppressive CD127(+) forkhead box P3 (FoxP3)(+) T cells into CD127(-) CD25(high) FoxP3(+) regulatory T cells. Clin. Exp. Immunol. 2011, 165, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Waickman, A.T.; Keller, H.R.; Kim, T.H.; Luckey, M.A.; Tai, X.; Hong, C.; Molina-París, C.; Walsh, S.T.R.; Park, J.H. The Cytokine Receptor IL-7Rα Impairs IL-2 Receptor Signaling and Constrains the In Vitro Differentiation of Foxp3(+) Treg Cells. iScience 2020, 23, 101421. [Google Scholar] [CrossRef] [PubMed]
- Heninger, A.K.; Theil, A.; Wilhelm, C.; Petzold, C.; Huebel, N.; Kretschmer, K.; Bonifacio, E.; Monti, P. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J. Immunol. 2012, 189, 5649–5658. [Google Scholar] [CrossRef] [Green Version]






| Marker | Fluorochrome | Clone | Brand |
|---|---|---|---|
| Anti-CD4 | FITC | RPAT4 | BD Bioscience |
| Anti-CD25 | APC | M-A251 | BD Bioscience |
| Anti-CD127 | PE-Cy7 | REA614 | Miltenyi Biotec |
| Anti-FoxP3 | PE | 259D/C7 | BD Bioscience |
| Fixable Viability dye | eF780 | eBioscience | |
| Violet Proliferation dye | VPD450 | eBioscience |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiori, A.; Uhlig, S.; Klüter, H.; Bieback, K. Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells. Cells 2021, 10, 58. https://doi.org/10.3390/cells10010058
Fiori A, Uhlig S, Klüter H, Bieback K. Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells. Cells. 2021; 10(1):58. https://doi.org/10.3390/cells10010058
Chicago/Turabian StyleFiori, Agnese, Stefanie Uhlig, Harald Klüter, and Karen Bieback. 2021. "Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells" Cells 10, no. 1: 58. https://doi.org/10.3390/cells10010058