An Agronomic Evaluation of New Safflower (Carthamus tinctorius L.) Germplasm for Seed and Oil Yields under Mediterranean Climate Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characterization and Main Cultivation Practices
2.2. Agronomic and Chemical Parameters
2.3. Statistical Analyses
3. Results
3.1. Analysis of Rainfall and Temperature Trends in the Test Site
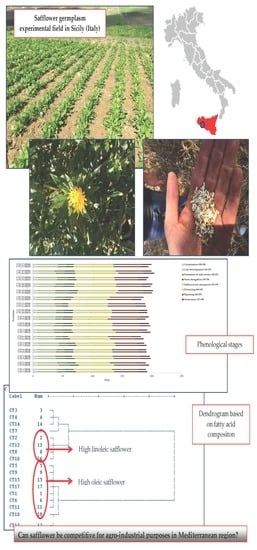
3.2. Growth Stages
3.3. Morphological and Yield Components Performance
3.4. Fatty Acid Composition and Qualitative Characteristics of Oils and Biomasses
3.5. Correlation and Linear Regression Analysis
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beyyavas, V.; Haliloglu, H.; Copur, O.; Yilmaz, A. Determination of seed yield and yield components of some safflower (Carthamus tinctorius L.) cultivars, lines and populations under the semi-arid conditions. Afr. J. Biotechnol. 2011, 10, 527–534. [Google Scholar] [CrossRef]
- El-Lattief, E.A. Evaluation of 25 safflower genotypes for seed and oil yields under arid environment in upper Egypt. Asia J. Crop Sci. 2012, 1–8. [Google Scholar] [CrossRef]
- Cho, M.H.; Paik, Y.S.; Hahn, T.R. Enzymatic conversion of pre-carthamin to carthamin by purified enzyme from the yellow petals of safflower. J. Agric. Food Chem. 2000, 48, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Corleto, A. Safflower project: On-farm introduction of safflower as an alternative oil crop in Southern Italy. In Proceedings of the Vth International Safflower Conference, Williston, ND, USA, 23–27 July 2001; Bergman, J.W., Műndel, H.H., Eds.; North Dakota State University: Fargo, ND, USA, 2001; pp. 179–184. [Google Scholar]
- Omidi, A.H.; Khazaei, H.; Hongbo, S. Variation for some important agronomic traits in 100 spring safflower (Carthamus tinctorius L.) genotypes. Am.-Eur. J. Agric. Environ. Sci. 2009, 5, 791–795. [Google Scholar]
- Fatahi, N.; Carapetian, J.; Heidari, R. Spectrophotometric measurement of valuable pigments from petals of safflower (Carthamus tinctorius L.) and their identification by TLC method. Res. J. Biol. Sci. 2008, 3, 761–763. [Google Scholar]
- Danieli, P.P.; Primi, R.; Ronchi, B.; Ruggeri, R.; Rossini, F.; Del Puglia, S.; Cereti, C.F. The potential role of spineless safflower (Carthamus tinctorius L. var. inermis) as fodder crop in central Italy. Ital. J. Agron. 2011, 6, 19–22. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Miranda, I.; Pereira, H. Evaluation of oil composition of some crops suitable for human nutrition. Ind. Crop. Prod. 2006, 24, 75–78. [Google Scholar] [CrossRef]
- Khan, M.A.; von Witzke-Ehbrecht, S.; Maass, B.L.; Becker, H.C. Relationships among different geographical groups, agro-morphology, fatty acid composition and RAPD marker diversity in Safflower (Carthamus tinctorius). Genet. Resour. Crop Evol. 2009, 56, 19–30. [Google Scholar] [CrossRef]
- Rudolphi, S.; Becker, H.C.; Schierolt, A.; von Witzke-Ehbrecht, S. Improved estimation of oil, linoleic and oleic acid and seed hull fractions in safflower by NIRS. J. Am. Oil Chem. Soc. 2012, 89, 363–369. [Google Scholar] [CrossRef]
- Ekin, Z. Resurgence of Safflower (Carthamus tinctorius L.) utilization: A global view. J. Agron. 2005, 4, 83–87. [Google Scholar] [CrossRef]
- de Oliveira, C.V.K.; Santos, R.F.; Siqueira, J.A.C.; Bariccatti, R.A.; Lenz, N.B.G.; Cruz, G.S.; Tokura, L.K.; Klajn, F.F. Chemical characterization of oil and biodiesel from four safflower genotypes. Ind. Crop. Prod. 2018, 123, 192–196. [Google Scholar] [CrossRef]
- Ҫamaş, N.; Ҫirak, C.; Esendal, E. Seed yield, oil content and fatty acid composition of safflower (Carthamus tinctorius L.) grown in northern Turkey conditions. J. Fac. Agric. OMU 2007, 22, 98–104. [Google Scholar]
- Coşge, B.; Gurbuz, B.; Kiralan, M. Oil content and fatty acid composition of some safflower (Carthamus tinctorius L.) varieties sown in spring and winter. Int. J. Nat. Eng. Sci. 2007, 1, 11–15. [Google Scholar]
- Anjani, K.; Yadav, P. High yielding-high oleic non-genetically modified Indian safflower cultivars. Ind. Crop. Prod. 2017, 104, 7–12. [Google Scholar] [CrossRef]
- Velasco, L.; Fernández-Martínez, J. Breeding for oil quality in Safflower. In Proceedings of the Vth International Safflower Conference, Williston, ND, USA, 23–27 July 2001; Bergman, J.W., Műndel, H.H., Eds.; North Dakota State University: Fargo, ND, USA, 2001; pp. 133–137. [Google Scholar]
- Hamdan, Y.A.S.; Perez-Vich, B.; Fernandez-Martinez, J.M.; Velasco, L. Inheritance of very high linoleic acid content and its relationship with nuclear male sterility in safflower. Plant Breed. 2008, 127, 507–509. [Google Scholar] [CrossRef]
- Galavi, M.; Romroudi, M.; Tavassoli, A. Effect of micronutrientes foliar application on yield and seed oil content of safflower (Carthamus tinctorius). Afr. J. Agric. Res. 2012, 7, 482–486. [Google Scholar]
- Knowles, P.F.; Hill, A.B. Inheritance of fatty acid content in the seed oil of a safflower introduction from Iran. Crop Sci. 1964, 4, 406–409. [Google Scholar] [CrossRef]
- Gecgel, U.; Demirci, M.; Esendal, E.; Tasan, M. Fatty acid composition of the oil from developing seeds of different varieties of safflower (Carthamus tinctorius L.). J. Am. Oil Chem. Soc. 2007, 84, 47–54. [Google Scholar] [CrossRef]
- Fernández-Martínez, J.; Rio, M.D.; Haro, A.D. Survey of safflower (Carthamus tinctorius L.) germplasm for variants in fatty acid composition and other seed characters. Euphytica 1993, 69, 115–122. [Google Scholar] [CrossRef]
- Corleto, A.; Alba, E.; Polignano, G.B.; Vonghio, G. Safflower: A multipurpose species with unexploited potential and world adaptability. In Proceedings of the IVth International Safflower Conference, Bari, Italy, 2–7 June 1997; pp. 23–31. [Google Scholar]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M. Evaluating the contribution of ionic and agronomic components toward salinity tolerance in safflower. Agron. J. 2015, 107, 2205–2212. [Google Scholar] [CrossRef]
- Izquierdo, N.; Aguirrezabal, L.; Andrade, F.; Pereyra, V. Night temperature affects fatty acid composition in sunflower oil depending on the hybrid and the phenological stage. Field Crop. Res. 2002, 7, 115–126. [Google Scholar] [CrossRef]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M.; Fotovat, R.; Feizi, M.; Pourdad, S.S. Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chem. 2012, 130, 618–625. [Google Scholar] [CrossRef]
- Amini, H.; Arzani, A.; Karami, M. Effect of water deficiency on seed quality and physiological traits of different safflower genotypes. Turk. J. Biol. 2014, 38, 271–282. [Google Scholar] [CrossRef]
- Lazzeri, L.; D’Avino, L.; Mazzoncini, M.; Antichi, D.; Mosca, G.; Zanetti, F.; Del Gatto, A.; Pieri, S.; De Mastro, G.; Grassano, N.; et al. On farm agronomic and first environmnetal evaluation of oil crops for sustainable bioenergy chain. Ital. J. Agron. 2009, 4, 171–180. [Google Scholar] [CrossRef]
- Stamigna, C.; Chiaretti, D.; Chiaretti, E.; Prosini, P.P. Oil and furfural recovery from Brassica carinata. Biomass Bioenergy 2012, 39, 478–483. [Google Scholar] [CrossRef]
- Licata, M.; La Bella, S.; Lazzeri, L.; Matteo, R.; Leto, C.; Massaro, F.; Tuttolomondo, T. Agricultural feedstocks of two Brassica oilseed crops and energy cogeneration with pure vegetable oil for a sustainable short agro-energy chain in Sicily (Italy). Ind. Crop. Prod. 2018, 117, 140–148. [Google Scholar] [CrossRef]
- Montemurro, F.; Diacono, M.; Scarcella, M.; D’Andrea, L.; Boari, F.; Santino, A.; Mastrorilli, M. Agronomic performance for biodiesel production potential of Brassica carinata A. Braun in Mediterranean marginal areas. Ital. J. Agron. 2016, 5, 57–64. [Google Scholar] [CrossRef]
- Tavarini, S.; Angelini, L.G.; Casadei, N.; Spugnoli, P.; Lazzeri, L. Agronomical evaluation and chemical characterization of Linum usitatissimum L. as oilseed crop for bio-based products in two environments of Central and Northern Italy. Ital. J. Agron. 2016, 11, 122–132. [Google Scholar] [CrossRef]
- Licata, M.; La Bella, S.; Leto, C.; Bonsangue, G.; Gennaro, M.C.; Tuttolomondo, T. Agronomic evaluation of Ethiopian mustard (Brassica carinata A. Braun) germplasm and physical-energy characterization of crop residues in a semi-arid area of Sicily (Italy). Chem. Eng. Trans. 2017, 58, 535–540. [Google Scholar] [CrossRef]
- Spugnoli, P.; Dainelli, R.; D’Avino, L.; Mazzoncini, M.; Lazzeri, L. Sustainability of sunflower cultivation for biodiesel production in Tuscany within the EU Renewable Energy Directive. Biosyst. Eng. 2012, 112, 49–55. [Google Scholar] [CrossRef]
- Servizio Informativo Agrometeorologico Siciliano. Available online: www.sias.regione.sicilia.it (accessed on 21 September 2015).
- Lancashire, P.D. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- ISO 22630 2015. Oilseed Meals—Determination of Oil Content—Rapid Extraction Method; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Mariotti, F.; Tomè, D.; Mirand, P.P. Converting nitrogen into protein-beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Maehre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein determination-Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef]
- Conte, L.S.; Leoni, O.; Palmieri, S.; Capella, P.; Lercker, G. Half-seed analysis: Rapid chromatographic determination of the main fatty acids of sunflower seed. Plant Breed. 1989, 102, 158–165. [Google Scholar] [CrossRef]
- ISO 12966-4 2015. Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Alizadeh, H.; Jirair, C. Genetic variation in a safflower germplasm grown in rainfed cold drylands. J. Agron. 2006, 5, 50–52. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Cultivar and seasonal effects on the contribution of pre-anthesis assimilates to safflower yield. Field Crop Res. 2004, 90, 263–274. [Google Scholar] [CrossRef]
- Bagawan, I.; Ravikumar, R.L. Strong undesirable linkages between seed yield and oil components—A problem in safflower improvement. In Proceedings of the Vth International Safflower Conference, Williston, ND, USA, 23–27 July 2001; Bergman, J.W., Műndel, H.H., Eds.; North Dakota State University: Fargo, ND, USA, 2001; pp. 143–149. [Google Scholar]
- Alizadeh, K. Evaluation of safflower germplasm by some agronomic characteristics and their relationships on grain yield production in the cold dry land of Iran. Int. J. Agric. Biol. 2005, 3, 389–391. [Google Scholar]
- Cosentino, S.; Copani, V.; Camarata, M. Relations between meteorological parameters yield and seed oil content in safflower in Mediterranean environment. In Proceedings of the IVth International Safflower Conference, Bari, Italy, 2–7 June 1997; pp. 149–155. [Google Scholar]
- Mohammadi, M.; Ghassemi-Golezani, K.; Chaichi, M.R.; Safikhani, S. Seed oil accumulation and yield of safflower affected by water supply and harvest time. Agron. J. 2018, 110, 1–8. [Google Scholar] [CrossRef]
- Özel, A.; Demirbilek, T.; Gür, M.A.; Ҫopur, O. Effects of different sowing date and intrarow spacing on yield and some agronomic traits of safflower (Carthamus tinctorius L.) under Harran Plain’s arid conditions. Turk. J. Agric. For. 2004, 28, 413–419. [Google Scholar]
- Bassil, E.S.; Kaffka, S.R. Response of safflower (Carthamus tinctorius L.) to saline soils and irrigation: I. Consumptive water use. Agric. Water Manag. 2002, 54, 67–80. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, K. Effect of irrigation regimes on oil content and composition of safflower (Carthamus tinctorius L.) cultivars. J. Am. Oil Chem. Soc. 2010, 87, 499–506. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Papakosta, D.K. Adaptation, grain yield and oil content of safflower in Greece. In Proceedings of the VIth International Safflower Conference, Istanbul, Turkey, 6–10 June 2005; pp. 161–166. [Google Scholar]
- Zhang, Z.; Chen, T. Studies on ecological adaptability of safflower germplasms in Xinjiang, China. In Proceedings of the VIth International Safflower Conference, Istanbul, Turkey, 6–10 June 2005; pp. 132–138. [Google Scholar]
- Arslan, B.; Culpan, E. Identification of suitable safflower genotypes for the development of new cultivars with high seed yield, oil content and oil quality. Azarian J. Agric. 2018, 5, 133–141. [Google Scholar]
- Gawand, P.B.; Tambe, S.I.; Reddy, B.N. Evaluation of productivity of safflower cultivars under moinsture and nutrient management in rainfed vertisols. In Proceedings of the VIth International Safflower Conference, Istanbul, Turkey, 6–10 June 2005; pp. 205–210. [Google Scholar]
- Oz, M. Relationship between sowing time, variety, and quality in safflower. J. Chem. 2016, 1–8. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Leto, C.; Leone, R.; Licata, M.; Virga, G.; Ruberto, G.; Napoli, E.M.; La Bella, S. Essential oil characteristics of wild Sicilian oregano populations in relation to environmental conditions. J. Essent. Oil Res. 2014, 26, 210–220. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Leto, C.; Cicero, N.; Tropea, A.; Virga, G.; Leone, R.; Licata, M.; La Bella, S. Agronomical and chemical characterisation of Thymbra capitata (L.) Cav. biotypes from Sicily, Italy. Nat. Prod. Res. 2015, 29, 1289–1299. [Google Scholar] [CrossRef]
- Baydar, H.; Turgut, I. Variation of fatty acid composition according to some morphological and physiological properties and ecological regions in oilseed plants. Turk. J. Agric. For. 1999, 23, 81–86. [Google Scholar]


| Code | Spiny/Spineless | Origin |
|---|---|---|
| CTI 1 | Spiny | Bangladesh |
| CTI 2 | Spiny | Bangladesh |
| CTI 3 | Spiny | Bangladesh |
| CTI 4 | Spiny | USA |
| CTI 5 | Spiny | Bangladesh |
| CTI 6 | Spiny | USA |
| CTI 7 | Spiny | USA |
| CTI 8 | Spiny | Canada |
| CTI 9 | Spiny | USA |
| CTI 10 | Spiny | Bangladesh |
| CTI 11 1 | Spiny | Italy |
| CTI 12 | Spiny | India |
| CTI 13 | Spiny | USA |
| CTI 14 | Spiny | Bangladesh |
| CTI 15 | Spiny | India |
| CTI 16 | Spiny | USA |
| CTI 17 | Spiny | Italy |
| Factors | Plant Height (cm) | No. Branches | No. Capitula | TSW (g) | Seed Yield (t ha−1) | Oil Yield (t ha−1) | Oil Content (%) |
|---|---|---|---|---|---|---|---|
| Year | |||||||
| 2013 | 122.65 A | 13.41 A | 13.47 A | 39.29 A | 1.13 A | 0.40 A | 35.65 A |
| 2014 | 118.41 A | 12.86 A | 12.72 A | 39.02 A | 1.07 A | 0.37 A | 34.90 A |
| Accession | |||||||
| CTI 1 | 105.83 DE | 13.83 AB | 13.00 AB | 38.33 B | 1.17 ABC | 0.42 ABC | 35.66 BCD |
| CTI 2 | 137.83 AB | 13.51 AB | 12.00 AB | 38.05 B | 1.15 ABC | 0.32 BC | 27.50 EF |
| CTI 3 | 127.67 BC | 14.62 A | 14.33 A | 39.80 AB | 1.33 AB | 0.40 ABC | 29.83 EF |
| CTI 4 | 120.50 BCDE | 14.28 A | 14.48 A | 40.75 AB | 1.37 AB | 0.45 ABC | 33.01 CDE |
| CTI 5 | 105.83 DE | 12.05 AB | 10.65 B | 38.15 B | 0.92 BC | 0.39 ABC | 42.17 A |
| CTI 6 | 109.83 CDE | 13.11 AB | 12.58 AB | 39.16 AB | 1.23 ABC | 0.51 AB | 41.17 AB |
| CTI 7 | 103.83 E | 11.01 B | 11.45 AB | 36.66 B | 0.95 BC | 0.38 ABC | 39.67 AB |
| CTI 8 | 128.67 BC | 14.50 A | 16.00 A | 41.10 AB | 1.45 AB | 0.40 ABC | 27.33 EF |
| CTI 9 | 120.01 BCDE | 13.50 AB | 13.63 AB | 38.00 B | 1.25 ABC | 0.50 AB | 40.16 AB |
| CTI 10 | 101.16 E | 11.66 B | 11.50 AB | 39.33 AB | 0.91 BC | 0.38 ABC | 41.83 A |
| CTI 11 | 113.67 CDE | 13.31 AB | 12.66 AB | 39.65 AB | 1.20 ABC | 0.46 ABC | 38.17 ABC |
| CTI 12 | 149.17 A | 12.66 AB | 12.73 AB | 39.83 AB | 1.10 ABC | 0.29 C | 26.50 F |
| CTI 13 | 118.83 BCDE | 13.50 AB | 13.75 AB | 38.33 B | 0.83 BC | 0.31 BC | 37.83 ABC |
| CTI 14 | 124.01 BCD | 11.93 B | 11.83 AB | 37.41 B | 0.83 BC | 0.23 C | 28.01 EF |
| CTI 15 | 127.17 BC | 14.25 A | 14.50 A | 38.13 B | 1.25 ABC | 0.54 A | 43.16 A |
| CTI 16 | 117.83 BCDE | 11.00 B | 11.00 B | 40.78 AB | 0.70 C | 0.22 C | 31.83 DEF |
| CTI 17 | 137.67 AB | 14.65 A | 16.00 A | 43.25 A | 1.64 A | 0.59 A | 35.83 BCD |
| Fatty Acids | Group 1 (n = 4) | Group 2 (n = 4) | Group 3 (n = 8) | Group 4 (n = 1) |
|---|---|---|---|---|
| Arachidic acid | 0.15 ± 0.17 | 0.29 ± 0.03 | 0.44 ± 0.04 | 0.40 ± 0.02 |
| Behenic acid | 0.13 ± 0.15 | 0.15 ± 0.11 | 0.32 ± 0.04 | 0.30 ± 0.01 |
| Gadoleic acid | 0.05 ± 0.10 | 0.10 ± 0.10 | 0.26 ± 0.06 | 0.20 ± 0.00 |
| Linoleic acid | 58.79 ± 6.06 | 73.88 ± 3.90 | 19.02 ± 3.77 | 32.10 ± 0.02 |
| Oleic acid | 32.41 ± 5.83 | 17.13 ± 3.70 | 72.00 ± 3.80 | 58.80 ± 0.01 |
| Palmitic acid | 5.95 ± 0.26 | 5.96 ± 0.21 | 5.26 ± 0.15 | 5.40 ± 0.03 |
| Stearic acid | 2.26 ± 0.14 | 2.32 ± 0.12 | 2.27 ± 0.24 | 2.30 ± 0.01 |
| Others | 0.26 ± 0.08 | 0.16 ± 0.10 | 0.42 ± 0.20 | 0.50 ± 0.03 |
| Accession | C (% DM) | H (% DM) | N (% DM) | Protein (% DM) |
|---|---|---|---|---|
| CTI 1 | 61.17 ± 0.83 | 8.51 ± 0.44 | 2.89 ± 0.25 | 16.40 ± 1.84 |
| CTI 2 | 59.45 ± 0.15 | 7.93 ± 0.24 | 2.78 ± 0.27 | 18.61 ± 1.58 |
| CTI 3 | 59.55 ± 0.14 | 8.22 ± 0.30 | 2.55 ± 0.04 | 15.72 ± 0.08 |
| CTI 4 | 60.94 ± 0.46 | 8.44 ± 0.28 | 2.13 ± 0.12 | 13.71 ± 0.92 |
| CTI 5 | 64.65 ± 0.26 | 8.94 ± 0.52 | 3.45 ± 0.22 | 21.20 ± 1.52 |
| CTI 6 | 61.01 ± 0.01 | 8.50 ± 0.33 | 2.66 ± 0.15 | 16.31 ± 0.99 |
| CTI 7 | 62.43 ± 0.16 | 8.61 ± 0.30 | 2.89 ± 0.11 | 19.11 ± 0.77 |
| CTI 8 | 60.90 ± 0.41 | 7.76 ± 0.29 | 2.36 ± 0.24 | 14.71 ± 0.02 |
| CTI 9 | 61.94 ± 0.24 | 8.81 ± 0.35 | 2.91 ± 0.15 | 17.41 ± 1.44 |
| CTI 10 | 63.48 ± 0.25 | 8.99 ± 0.29 | 3.04 ± 0.10 | 19.72 ± 0.92 |
| CTI 11 | 61.90 ± 0.09 | 8.71 ± 0.36 | 1.99 ± 0.05 | 12.31 ± 0.38 |
| CTI 12 | 58.65 ± 0.73 | 8.01 ± 0.24 | 2.53 ± 0.10 | 16.31 ± 0.01 |
| CTI 13 | 61.28 ± 0.01 | 8.20 ± 0.18 | 2.82 ± 0.27 | 18.50 ± 1.91 |
| CTI 14 | 61.13 ± 0.12 | 8.09 ± 0.17 | 2.69 ± 0.06 | 17.31 ± 0.39 |
| CTI 15 | 62.27 ± 0.05 | 8.92 ± 0.37 | 3.65 ± 0.10 | 22.11 ± 0.36 |
| CTI 16 | 60.80 ± 0.40 | 8.20 ± 0.23 | 2.39 ± 0.11 | 15.91 ± 0.07 |
| CTI 17 | 61.23 ± 0.20 | 8.46 ± 0.28 | 2.44 ± 0.03 | 15.13 ± 0.19 |
| Accession | C (% DM) | H (% DM) | N (% DM) | |||
|---|---|---|---|---|---|---|
| AG 1 | BG 2 | AG | BG | AG | BG | |
| CTI 1 | 47.33 ± 1.20 | 48.43 ± 0.36 | 6.88 ± 0.62 | 6.21 ± 0.17 | 1.01 ± 0.12 | 0.60 ± 0.16 |
| CTI 2 | 47.73 ± 0.32 | 48.10 ± 0.71 | 6.66 ± 0.01 | 6.17 ± 0.07 | 0.91 ± 0.01 | 0.49 ± 0.03 |
| CTI 3 | 49.11 ± 0.86 | 47.44 ± 0.32 | 6.83 ± 0.24 | 6.15 ± 0.01 | 0.89 ± 0.15 | 0.59 ± 0.11 |
| CTI 4 | 48.43 ± 0.31 | 46.40 ± 0.11 | 6.72 ± 0.03 | 6.09 ± 0.01 | 0.83 ± 0.02 | 0.55 ± 0.01 |
| CTI 5 | 48.17 ± 0.01 | 45.68 ± 0.75 | 6.75 ± 0.02 | 5.82 ± 0.21 | 1.23 ± 0.10 | 0.81 ± 0.01 |
| CTI 6 | 47.55 ± 0.32 | 48.84 ± 0.61 | 6.55 ± 0.17 | 6.35 ± 0.06 | 0.86 ± 0.05 | 0.64 ± 0.04 |
| CTI 7 | 47.97 ± 0.39 | 44.46 ± 0.69 | 6.68 ± 0.03 | 5.95 ± 0.28 | 0.91 ± 0.05 | 0.44 ± 0.08 |
| CTI 8 | 48.13 ± 0.69 | 45.89 ± 0.51 | 6.59 ± 0.09 | 6.01 ± 0.07 | 0.65 ± 0.04 | 0.36 ± 0.01 |
| CTI 9 | 48.08 ± 0.07 | 48.81 ± 0.96 | 6.67 ± 0.03 | 6.35 ± 0.06 | 1.02 ± 0.17 | 0.61 ± 0.03 |
| CTI 10 | 48.28 ± 0.36 | 47.46 ± 0.39 | 6.52 ± 0.15 | 6.12 ± 0.07 | 0.94 ± 0.04 | 0.66 ± 0.08 |
| CTI 11 | 48.27 ± 0.94 | 42.74 ± 0.59 | 6.57 ± 0.11 | 5.56 ± 0.09 | 0.56 ± 0.02 | 0.43 ± 0.07 |
| CTI 12 | 47.57 ± 0.37 | 47.29 ± 0.51 | 6.51 ± 0.05 | 6.27 ± 0.02 | 0.97 ± 0.01 | 0.59 ± 0.01 |
| CTI 13 | 48.40 ± 0.37 | 48.27 ± 0.73 | 6.29 ± 0.20 | 6.12 ± 0.10 | 1.02 ± 0.01 | 0.68 ± 0.02 |
| CTI 14 | 47.85 ± 0.16 | 46.75 ± 0.81 | 6.39 ± 0.08 | 6.08 ± 0.10 | 0.96 ± 0.18 | 0.59 ± 0.01 |
| CTI 15 | 48.46 ± 0.75 | 44.54 ± 0.75 | 6.70 ± 0.10 | 5.71 ± 0.08 | 1.12 ± 0.01 | 0.77 ± 0.13 |
| CTI 16 | 48.20 ± 0.69 | 47.08 ± 0.07 | 6.53 ± 0.09 | 6.18 ± 0.01 | 0.80 ± 0.03 | 0.36 ± 0.01 |
| CTI 17 | 48.67 ± 0.85 | 48.22 ± 0.44 | 6.45 ± 0.10 | 6.25 ± 0.04 | 0.87 ± 0.01 | 0.47 ± 0.06 |
| Traits | No. Branches | No. Capitula | TSW (g) | Seed Yield (t ha−1) | Oil Content (%) | Oil Yield (t ha−1) |
|---|---|---|---|---|---|---|
| No. branches (n) | 1.00 | |||||
| No. capitula (n) | 0.89 ** | 1.00 | ||||
| TSW (g) | 0.57 ** | 0.79 ** | 1.00 | |||
| Seed yield (t ha−1) | 0.90 ** | 0.96 ** | 0.79 ** | 1.00 | ||
| Oil content (%) | −0.16 | −0.21 | −0.21 | −0.13 | 1.00 | |
| Oil yield (t ha−1) | 0.67 ** | 0.67 ** | 0.54 ** | 0.73 ** | 0.52 ** | 1.00 |
| Independence Variable (X) | Dependence Variable (Y) | Regression Equation | R2 (%) |
|---|---|---|---|
| Plant height | Seed yield | Y = 0.82 + 0.09X | 17.61 ** |
| Oil yield | Y = 4.00 − 0.00X | 0.01 | |
| Number of branches | Seed yield | Y = −6.90 + 1.39X | 81.90 ** |
| Oil yield | Y = −1.81 + 0.45X | 45.10 ** | |
| Number of capitula | Seed yield | Y = −4.87 + 1.24X | 91.91 ** |
| Oil yield | Y = −0.87 + 0.38X | 44.92 ** | |
| TSW | Seed yield | Y = −41.77 + 1.35X | 62.82 ** |
| Oil yield | Y = −11.74 + 0.40X | 29.20 ** | |
| Oil content | Seed yield | Y = 13.33 − 0.06X | 1.81 |
| Oil yield | Y = 0.23 + 0.10X | 27.32 ** | |
| Oil yield | Seed yield | Y = 0.45 + 0.31X | 53.81 ** |
| Seed yield | Oil yield | Y = 4.39 + 1.68X | 53.82 ** |
| Variable | Eigenvectors | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| Plant height (cm) | 0.41 | 0.49 | 0.63 | −0.05 | 0.05 | 0.33 |
| No. branches | 0.86 | −0.07 | 0.28 | 0.23 | −0.16 | 0.06 |
| No. capitula | 0.94 | 0.15 | 0.09 | 0.18 | 0.60 | 0.11 |
| TSW (g) | 0.89 | 0.17 | −0.12 | −0.01 | 0.22 | 0.03 |
| Seed yield (t ha−1) | 0.98 | −0.02 | 0.08 | 0.10 | −0.01 | 0.03 |
| Oil yield (t ha−1) | 0.80 | −0.54 | −0.11 | 0.23 | −0.18 | 0.09 |
| Oil content (%) | −0.10 | −0.80 | −0.43 | −0.15 | −0.26 | 0.03 |
| Days to germination (n) | −0.20 | 0.75 | −0.11 | 0.01 | 0.88 | −0.23 |
| Days to leaf development (n) | 0.29 | −0.22 | −0.13 | −0.49 | −0.35 | 0.65 |
| Days to formation of side shoots (n) | −0.41 | 0.10 | 0.88 | −0.07 | −0.14 | 0.24 |
| Days to stem elongation (n) | −0.49 | 0.41 | −0.10 | 0.18 | 0.59 | 0.20 |
| Days to inflorescence emergence (n) | 0.08 | −0.70 | 0.07 | 0.10 | 0.45 | −0.14 |
| Days to flowering (n) | −0.07 | 0.38 | −0.15 | −0.46 | 0.09 | 0.94 |
| Days to ripening (n) | 0.32 | 0.09 | −0.15 | 0.83 | 0.20 | 0.05 |
| Days to senescence (n) | 0.10 | 0.05 | 0.88 | 0.28 | −0.02 | −0.17 |
| Oleic acid (%) | 0.13 | 0.95 | −0.19 | −0.10 | −0.08 | 0.08 |
| Linoleic acid (%) | −0.13 | 0.95 | 0.20 | 0.09 | 0.07 | −0.09 |
| Palmitic acid (%) | 0.25 | 0.82 | −0.25 | 0.13 | 0.11 | −0.06 |
| Stearic acid (%) | 0.14 | 0.14 | 0.37 | 0.83 | −0.13 | −0.09 |
| Eigenvalues | ||||||
| Total eigenvalue | 5.27 | 5.18 | 2.51 | 2.04 | 1.14 | 1.05 |
| Percent of total variance explained (%) | 27.73 | 27.30 | 13.20 | 10.74 | 5.98 | 5.52 |
| Cumulative percent of total variance explained (%) | 27.73 | 55.03 | 68.24 | 78.98 | 84.96 | 90.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Bella, S.; Tuttolomondo, T.; Lazzeri, L.; Matteo, R.; Leto, C.; Licata, M. An Agronomic Evaluation of New Safflower (Carthamus tinctorius L.) Germplasm for Seed and Oil Yields under Mediterranean Climate Conditions. Agronomy 2019, 9, 468. https://doi.org/10.3390/agronomy9080468
La Bella S, Tuttolomondo T, Lazzeri L, Matteo R, Leto C, Licata M. An Agronomic Evaluation of New Safflower (Carthamus tinctorius L.) Germplasm for Seed and Oil Yields under Mediterranean Climate Conditions. Agronomy. 2019; 9(8):468. https://doi.org/10.3390/agronomy9080468
Chicago/Turabian StyleLa Bella, Salvatore, Teresa Tuttolomondo, Luca Lazzeri, Roberto Matteo, Claudio Leto, and Mario Licata. 2019. "An Agronomic Evaluation of New Safflower (Carthamus tinctorius L.) Germplasm for Seed and Oil Yields under Mediterranean Climate Conditions" Agronomy 9, no. 8: 468. https://doi.org/10.3390/agronomy9080468